Predicting Products | Single Replacement Reactions
TLDRThis video script offers a comprehensive guide on identifying and predicting the outcomes of single replacement reactions. It explains the common pattern where an element reacts with a compound, resulting in a new element and compound. The video distinguishes between reactions involving two metals, where the more reactive metal displaces the less reactive one, and those involving halogens, where the more reactive halogen replaces the less reactive one in a compound. Utilizing the activity series as a reference, the script provides a step-by-step method for predicting products, emphasizing the importance of understanding the reactivity series and the need to account for diatomic elements in the process.
Takeaways
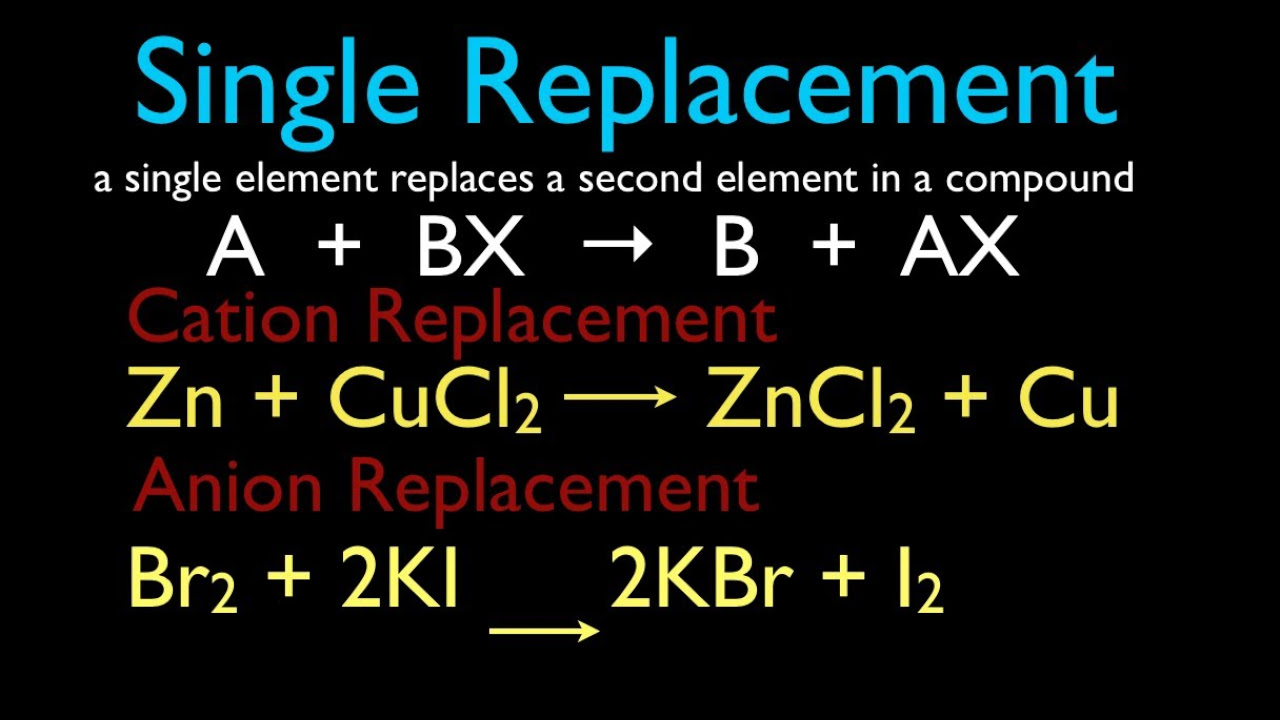
- 🧪 In a single replacement reaction, an element reacts with a compound to form a new element and a new compound.
- 🔄 The two categories of single replacement reactions involve either two metals switching places or two halogens replacing each other.
- 🏅 The activity series is a crucial reference for predicting whether a single replacement reaction will occur, with metals and halogens listed in order of reactivity.
- 🌟 For metals, the lone element must be more reactive (higher on the activity series) than the metal in the compound to displace it.
- 💧 In the case of halogens, the lone halogen must be more reactive (higher on the activity series) than the halogen in the compound to replace it.
- 📊 The activity series can be found on reference sheets or online resources for use in predicting reaction outcomes.
- 🤹♂️ When writing products for ionic compounds, it's essential to crisscross the charges to determine the correct formula.
- 📚 Some elements, like hydrogen, act as metals in single replacement reactions, though they are nonmetals in other contexts.
- 🔢 Diatomic elements, such as hydrogen (H2), fluorine (F2), and bromine (Br2), need to be recognized and written correctly in the products.
- 🛑 If the lone element or halogen is not higher on the activity series, no reaction will occur, and the original compound remains unchanged.
Q & A
What pattern is commonly seen in a single replacement reaction?
-In a single replacement reaction, the common pattern observed is an element reacting with a compound, resulting in an element by itself and a new compound.
How can you distinguish between the two categories of single replacement reactions?
-The two categories of single replacement reactions can be distinguished based on whether the reactants include two metals or two halogens. Metals switch places with metals, and halogens switch places with halogens.
What role does the activity series play in predicting the outcome of a single replacement reaction?
-The activity series determines whether the single replacement reaction will occur by indicating if the lone element (metal or halogen) is more reactive than the element in the compound. The lone element must be higher on the activity series to replace the element in the compound.
Why is bromine written as Br2 instead of Br when alone in a single replacement reaction?
-Bromine is written as Br2 instead of Br because it is a diatomic element, meaning it is more stable in its diatomic form (two atoms bonded together) when it is by itself.
What happens if the lone element is not higher on the activity series than the element in the compound?
-If the lone element is not higher on the activity series than the element in the compound, the reaction does not occur, and it is indicated that there is 'no reaction.'
How do you predict the new compound formed in a single replacement reaction involving metals?
-To predict the new compound formed when metals are involved, you identify the metal replacing the original metal in the compound. Then, you need to crisscross the charges of the ions to ensure the formula is correctly balanced.
In the context of single replacement reactions, what is meant by 'crisscrossing' charges?
-Crisscrossing charges refers to the method of using the charge of one ion as the subscript for the other ion and vice versa to balance the charges and create a neutral compound. This method is used when forming new ionic compounds.
What is a key difference in predicting the outcome of reactions involving halogens compared to those involving metals?
-The key difference in predicting the outcome of reactions involving halogens, as opposed to metals, lies in referring to the halogen side of the activity series to see if the lone halogen is more reactive than the halogen in the compound.
Why is it important to know the diatomic elements when predicting products of single replacement reactions?
-Knowing the diatomic elements is important because if they are produced as a product in a single replacement reaction, they must be written in their diatomic form (e.g., H2, O2, N2) to accurately represent their stable, naturally occurring state.
How does the reactivity of hydrogen in single replacement reactions differ from its usual classification as a nonmetal?
-In the context of single replacement reactions, hydrogen can act like a metal, meaning it can be replaced by a metal higher on the activity series, even though it is generally classified as a nonmetal.
Outlines
🔍 Identifying Single Replacement Reactions
This paragraph introduces the concept of single replacement reactions, explaining how to recognize them by looking for an element reacting with a compound. It highlights two categories: reactions involving two metals where the element will replace the metal in the compound, and reactions involving two halogens where they will replace each other. The importance of the activity series is introduced as a tool to predict whether a reaction will occur based on the relative positions of the elements or halogens involved.
🧪 Predicting Products and the Role of the Activity Series
The paragraph delves into the process of predicting the products of single replacement reactions using the activity series. It explains how to determine if a reaction will occur by checking if the lone element (metal or halogen) is higher on the activity series than the element in the compound. The paragraph provides examples of reactions with different combinations of metals and halogens, illustrating how to predict the outcome based on their positions in the activity series.
📝 Practice Problems for Predicting Reaction Products
This paragraph presents a series of practice problems to apply the knowledge of single replacement reactions. It guides the viewer through identifying the type of reaction (metal with compound or halogen with halogen) and using the activity series to predict the products. The paragraph emphasizes the need to remember diatomic elements and the process of crisscrossing charges when writing the formulas for the new compounds formed.
Mindmap
Keywords
💡Single Replacement Reaction
💡Activity Series
💡Diatomic Element
💡Compound
💡Element
💡Ionic Bonding
💡Halogen
💡Metal
💡Predicting Reaction Products
💡No Reaction Condition
Highlights
Introduction to predicting products of a single replacement reaction and recognizing such reactions from reactants.
Explanation of the common pattern in single replacement reactions: an element reacting with a compound.
Illustration of a metal-metal replacement example with calcium and magnesium.
Introduction to halogen-halogen replacement reactions with an example of aluminum chloride and bromine.
Explanation of the significance of the activity series in predicting reaction outcomes.
Demonstration of how the activity series determines whether a metal can replace another metal.
Explanation of why bromine cannot replace chlorine in a compound due to its position in the activity series.
Overview of practice problems to predict products of single replacement reactions.
Details on identifying a single replacement reaction from reactants.
Criteria for determining the outcome of metal-metal and halogen-halogen replacement reactions.
Explanation and application of the crisscross method for forming new compounds.
Identification and replacement process of halogens in a compound, including diatomic stability considerations.
Methodology for predicting products when an element is reacting with a compound containing a halogen.
Special consideration of hydrogen as a metal in single replacement reactions.
Step-by-step guide for using the activity series to predict the outcome of single replacement reactions.
Final summary and encouragement for practice to master predicting products of single replacement reactions.
Transcripts
Browse More Related Video

GCSE Chemistry - Reactivity Series of Metals & Displacement Reactions #37

BTEC Applied Science: Unit 1 Chemistry Reactions of Metals
Chemical Reactions (2 of 11) Single Replacement Reactions, An Explanation

Types of Chemical Reactions

SYNTHESIS REACTIONS

Single Replacement Reactions and Net Ionic Equations
5.0 / 5 (0 votes)
Thanks for rating: