Lec-16 I Introduction to phase rule I Applied chemistry
TLDRIn this video lecture, Sukruti Joshi from the Energy Institute of Engineering and Technology introduces Applied Chemistry, subject code 3130506. The lecture covers various chapters including physical properties, organic reactions, stereochemistry, quantum chemistry, and coordination chemistry. The focus of the session is on the Phase Rule (Gibbs Phase Rule), its equation (f = c - p + 2), and its significance in understanding changes in matter's state due to temperature and pressure. The lecture also discusses the applications of the Phase Rule in predicting equilibrium changes in heterogeneous systems and its importance in metallurgical operations and alloy formation.
Takeaways
- 📚 The lecture series is on Applied Chemistry with the subject code 3130506, presented by Sukruti Joshi from the Energy Institute of Engineering and Technology, Ahmed.
- 🌟 Previous sessions covered topics like physical properties of matter, general principles in organic reactions, stereochemistry, quantum chemistry, and coordination chemistry.
- 📈 Chapter 5 focuses on the Phase Rule, which is essential for understanding changes in the state of matter under varying conditions such as temperature and pressure.
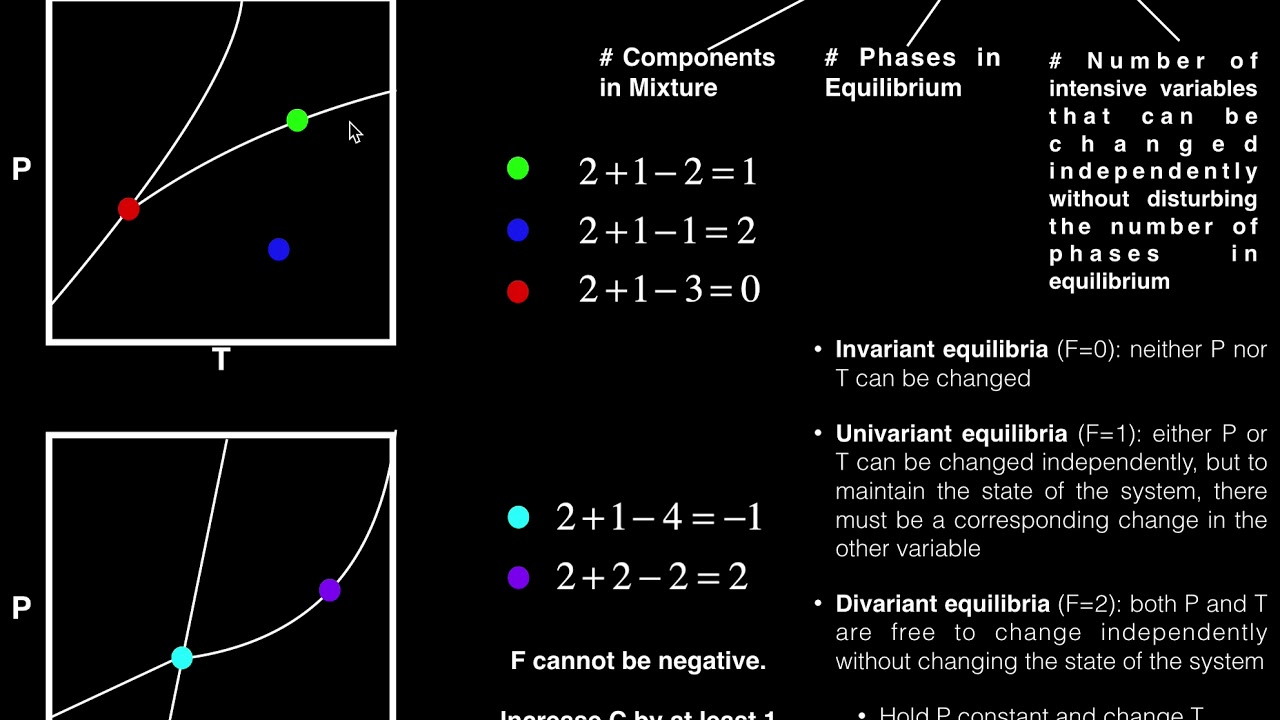
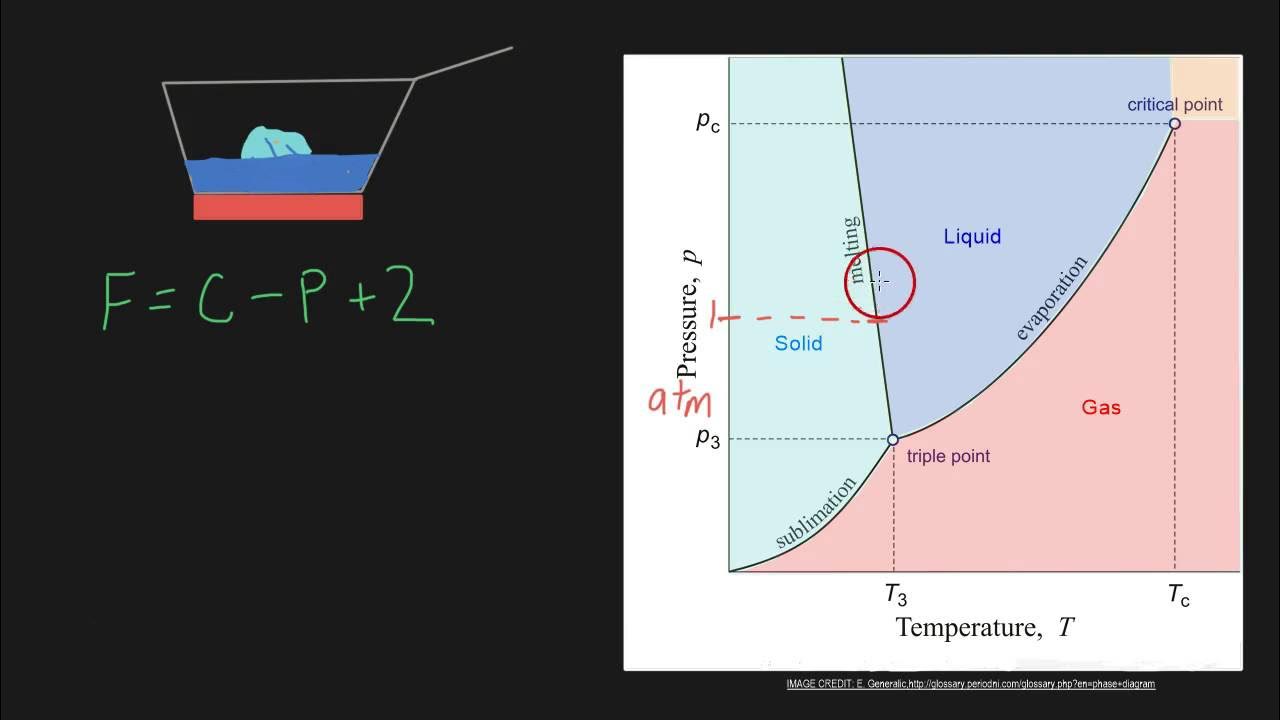
- 🔍 The Phase Rule is introduced by the equation f = c - p + 2, where f represents degrees of freedom, c is the number of components, and p is the number of phases.
- 🌐 The concept of 'phase' refers to any homogeneous part of a system with uniform physical and chemical properties throughout.
- 🔥 The four states of matter are solid, liquid, gas, and plasma, with the latter existing under specific conditions.
- 🌡️ Changes in the state of matter, such as melting or vaporization, occur due to changes in temperature or pressure.
- 🔄 The Phase Rule, discovered by American physicist Ellard in 1874 and later refined by Willard Gibbs, helps predict the effects of changing pressure, temperature, and concentration on a system in equilibrium.
- 🏭 The rule is widely applied in metallurgical operations for understanding the formation of alloys at specific temperatures, pressures, and concentrations.
- 📊 The Phase Rule allows for the qualitative prediction of how equilibrium in a heterogeneous system is affected by changes in external factors.
- 🎓 A single-phase system is considered homogeneous, while a system with two or more phases in contact is a heterogeneous system.
Q & A
Who is the speaker in the video lecture and what is their field of expertise?
-The speaker in the video lecture is Sukruti Joshi, and their field of expertise is Applied Chemistry.
What is the subject code for Applied Chemistry in the lecture series?
-The subject code for Applied Chemistry in the lecture series is 3130506.
What are the different chapters covered in the previous sessions of Applied Chemistry?
-In the previous sessions, the chapters covered include physical properties and their relation to constitution, general principles involved in organic reactions, main types of organic reactions, stereochemistry, quantum chemistry, and coordination chemistry.
What is the topic of the fifth chapter in the Applied Chemistry series?
-The topic of the fifth chapter in the Applied Chemistry series is the Phase Rule.
Who is the scientist that developed the equation for the Phase Rule?
-The scientist who developed the equation for the Phase Rule is Willard Gibbs.
What does the Phase Rule state in terms of the relationship between degrees of freedom, components, and phases?
-The Phase Rule states that the degrees of freedom (F) in a chemical system are equal to the number of components (C) minus the number of phases (P) plus 2.
What is the significance of the Phase Rule in the study of chemistry?
-The Phase Rule is significant because it allows scientists to predict the effects of changing pressure, temperature, and concentration on a heterogeneous system in equilibrium.
In what type of systems can the Phase Rule be applied?
-The Phase Rule can be applied in systems that involve changes in the physical states of matter, such as solid, liquid, and gas, as well as in metallurgical operations and the study of alloys.
How many phases are there in a system containing only liquid water?
-In a system containing only liquid water, there is one phase, making it a single-phase or homogeneous system.
What constitutes a heterogeneous system in the context of the Phase Rule?
-A heterogeneous system, in the context of the Phase Rule, is a system that contains two or more phases in contact with each other or in equilibrium.
What is the definition of a phase according to the script?
-A phase is defined as any homogeneous part of a system that has all physical and chemical properties similar or the same throughout.
Outlines
📚 Introduction to Applied Chemistry and Phase Rule
This paragraph introduces the speaker, Sukruti Joshi, and the topic of the video lecture series on Applied Chemistry with subject code 3130506. It provides a brief overview of the previous sessions, including physical properties, organic reactions, stereochemistry, quantum chemistry, and coordination chemistry. The main focus of this session is to discuss the Phase Rule, a fundamental concept in understanding the equilibrium of chemical systems. The Phase Rule, also known as Gibbs Phase Rule, is introduced as a relationship between the degrees of freedom (F), the number of components (C), and the number of phases (P) in a system, represented by the equation F = C - P + 2. The importance of understanding the Phase Rule for predicting the effects of changes in pressure, temperature, and concentration on the equilibrium of heterogeneous systems is highlighted.
🔬 Historical Background and Applications of the Phase Rule
This paragraph delves into the history of the Phase Rule, which was first discovered in 1874 by American physicist Ellard. It emphasizes the significance of the Phase Rule in predicting the behavior of chemical systems under varying conditions, such as changes in pressure, temperature, and concentration. The applications of the Phase Rule are vast, particularly in metallurgical operations where it aids in understanding the formation of alloys at specific temperatures and pressures. The paragraph also clarifies the definition of a phase as a homogeneous part of a system with uniform physical and chemical properties throughout. Examples are provided to illustrate one-phase, two-phase, and three-phase systems, demonstrating how changes in physical properties lead to different phases and the overall classification of systems as homogeneous or heterogeneous.
🌟 Understanding Phases and Heterogeneous Systems
The final paragraph of the script focuses on the concept of phases and heterogeneous systems. It explains that a system containing only one phase is considered homogeneous, while a system with two or more phases in contact is termed heterogeneous. The distinction between homogeneous and heterogeneous systems is crucial for applying the Phase Rule. The paragraph concludes by summarizing the key points discussed in the lecture and teases the next session, which will continue exploring the Phase Rule and its implications in greater detail.
Mindmap
Keywords
💡Applied Chemistry
💡Physical Properties
💡Organic Reactions
💡Stereochemistry
💡Quantum Chemistry
💡Coordination Chemistry
💡Phase Rule
💡Degrees of Freedom
💡Components
💡Phases
💡Heterogeneous System
Highlights
Introduction to Applied Chemistry by Sukruti Joshi from Energy Institute of Engineering and Technology.
Subject code for Applied Chemistry is 3130506.
Review of previous sessions on physical properties, organic reactions, and stereochemistry.
Detailed study on quantum chemistry and coordination chemistry in chapter four.
Introduction to chapter five, focusing on the phase rule.
Definition of a phase in chemistry as solid, liquid, and gas states.
Existence of the fourth state of matter, plasma, under specific conditions.
Explanation of how temperature and pressure changes can convert phases of matter.
Discussion on the theory and branch of study dealing with phase changes.
Introduction to Willard Gibbs and the equation for the phase rule.
Gibbs Phase Rule formula: capital F = c - p + 2.
Historical context of the phase rule discovery by American physicist Ellard in 1874.
Applications of Gibbs Phase Rule in predicting qualitative changes in heterogeneous systems.
Use of phase rule in understanding changes in concentration, pressure, and temperature on equilibrium.
Importance of phase rule in metallurgical operations and alloy formation.
Definition of a phase as a homogeneous part of a system with uniform physical and chemical properties.
Examples of one, two, and three-phase systems and their physical property distinctions.
Differentiation between homogeneous and heterogeneous systems based on the number of phases.
Transcripts
Browse More Related Video

Lec-18 I The water system I Applied Chemistry I Chemical Engineering

Lec-21 I Eutectic systems I Applied chemistry I Chemical Engineering

Lec-01 I Introduction to Applied chemistry I Applied Chemistry I Chemical Engineering
Phase Diagrams | Gibbs Phase Rule (w/ 5 Examples)
Gibbs Phase Rule

Gibbs’ phase rule
5.0 / 5 (0 votes)
Thanks for rating: