17.1 Buffers and Buffer pH Calculations | General Chemistry
TLDRThis educational video delves into the concept of buffers, detailing their composition and function in resisting pH changes. It explains the importance of buffers in chemical reactions and biological systems, emphasizing their role in maintaining the narrow pH range essential for life. The video outlines the creation of buffers using weak acids with their conjugate bases or weak bases with their conjugate acids, and discusses the calculation of buffer pH using the Henderson-Hasselbalch equation. Practical examples and potential applications in both academic and biochemistry contexts are provided, highlighting the significance of buffers in scientific studies and real-world scenarios.
Takeaways
- 📚 A buffer is a solution that resists changes in pH, composed of a weak acid and its conjugate base or a weak base and its conjugate acid.
- 🧪 The pH stability of buffers is crucial for many chemical reactions and biological processes, including those in living organisms where pH must remain within a narrow range.
- 🔄 Buffers work by neutralizing added acids or bases, protecting water from being converted into H3O+ or OH-, thus maintaining the pH of the solution.
- 📈 The Henderson-Hasselbalch equation is a fundamental tool for buffer calculations, derived from the Ka expression, and allows for easy pH determination in buffer solutions.
- 🌡️ At a 1:1 ratio of weak acid to conjugate base, the pH of a buffer solution equals the pKa of the weak acid.
- 🔢 The pH of a buffer solution can be calculated by taking the pKa, and adjusting it based on the ratio of the conjugate base to the weak acid present in the solution.
- 💧 Adding strong acids or bases to a buffer solution results in a minimal change in pH due to the neutralization reaction that occurs, keeping the solution within its buffer range.
- 🧪 When calculating the pH of a buffer after adding a strong acid or base, it is important to account for the change in moles of the conjugate base and weak acid due to the neutralization reaction.
- 📊 The buffer range is typically around the pKa plus or minus one, indicating the pH range within which the buffer can effectively resist changes.
- 🔧 Polyprotic acids, which can donate more than one proton, have multiple pKas and can create multiple buffer ranges, offering versatility in buffering solutions for different pH environments.
- 🧬 Carbonic acid, a diprotic acid, plays a key role in the human blood buffering system, maintaining pH through the equilibrium with CO2 and H2O.
Q & A
What is a buffer solution and how does it resist changes in pH?
-A buffer solution is a mixture that resists changes in pH when small amounts of acids or bases are added. It consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. The buffer solution maintains the pH by reacting with added acids or bases, thus preventing significant changes in the pH level.
Why are buffer solutions important in chemical reactions and biological systems?
-Buffer solutions are crucial in chemical reactions and biological systems because many processes are pH-sensitive. They occur most efficiently within a narrow pH range. In living organisms, such as in human blood, buffers help maintain a stable pH, which is essential for survival.
What are the components of a buffer solution?
-A buffer solution is composed of a weak acid and its conjugate base, or a weak base and its conjugate acid. For example, a common buffer is a mixture of acetic acid (a weak acid) and sodium acetate (the conjugate base of acetic acid).
How does the Henderson-Hasselbalch equation help in calculating the pH of a buffer solution?
-The Henderson-Hasselbalch equation is a convenient way to calculate the pH of a buffer solution. It is derived from the Ka expression and relates the pH to the pKa of the weak acid, and the ratio of the concentrations of the conjugate base to the weak acid in the solution. The equation is: pH = pKa + log ([A-]/[HA]), where [A-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid.
What happens when a strong acid or base is added to a buffer solution?
-When a strong acid or base is added to a buffer solution, a neutralization reaction occurs. The strong acid will neutralize the base present (the conjugate base), converting it into the weak acid. Conversely, a strong base will neutralize the weak acid, converting it into the conjugate base. The buffer solution resists significant changes in pH due to this neutralization process.
What is the buffer range and how is it determined?
-The buffer range is the pH range around the pKa of the weak acid where the buffer solution is most effective at resisting changes in pH. It is typically around the pKa value, plus or minus one pH unit. The buffer range is determined by the ratio of the concentrations of the weak acid to its conjugate base.
How does the pH of a buffer solution change when strong acids or bases are added?
-The pH of a buffer solution changes very little when small amounts of strong acids or bases are added, due to the neutralization reaction that occurs. If a strong acid is added, the pH will decrease slightly but not by a large amount. If a strong base is added, the pH will increase slightly. The extent of the pH change depends on the amount and strength of the added acid or base and the buffer capacity.
What is the role of the conjugate acid and base in a buffer solution?
-In a buffer solution, the weak acid and its conjugate base work together to maintain a stable pH. When a strong acid is added, the conjugate base (A-) reacts with it to form more of the weak acid (HA), thus minimizing the increase in H+ concentration and preventing a significant decrease in pH. Conversely, when a strong base is added, the weak acid (HA) reacts to form more of the conjugate base (A-), minimizing the decrease in H+ concentration and preventing a significant increase in pH.
How can you calculate the pH of a buffer solution after the addition of a strong acid or base?
-To calculate the pH of a buffer solution after the addition of a strong acid or base, you first determine the limiting reagent in the neutralization reaction to find the new moles of the conjugate base and weak acid. Then, using the Henderson-Hasselbalch equation, you can calculate the new pH by plugging in the new moles of the conjugate base and weak acid, along with the pKa value of the weak acid.
What is the significance of the pKa value in the context of buffer solutions?
-The pKa value is the negative logarithm of the Ka (acid dissociation constant) of the weak acid in the buffer solution. It is a measure of the acid's strength and its tendency to donate a proton. In the context of buffer solutions, the pH of the solution is equal to the pKa when the concentrations of the weak acid and its conjugate base are equal. The pKa value is crucial for calculating the pH of a buffer solution and determining its buffering capacity.
What is the role of the Henderson-Hasselbalch equation in understanding buffer solutions?
-The Henderson-Hasselbalch equation provides a mathematical relationship that allows us to calculate the pH of a buffer solution. It simplifies the process by directly relating the pH to the pKa of the weak acid and the ratio of the concentrations of the conjugate base to the weak acid. This equation is particularly useful for quickly estimating the pH changes in buffer solutions upon the addition of strong acids or bases.
Outlines
📚 Introduction to Buffers and Chad's Prep
The video begins with an introduction to the topic of buffers, emphasizing their importance in maintaining pH levels, especially in chemical reactions and biological systems. The speaker, Chad, outlines the scope of the lesson, which includes general chemistry as well as specificDAT, MCAT, and OAT prep. He encourages viewers to subscribe and turn on notifications for updates. The lesson's main focus is to understand what buffers are, their composition (weak acid with its conjugate base or a weak base with its conjugate acid), and their role in resisting pH changes.
🧪 Buffer Composition and pH Resistance
This paragraph delves into the specifics of buffer composition, explaining that buffers are made up of a weak acid and its conjugate base or a weak base and its conjugate acid. The discussion highlights the importance of maintaining a narrow pH range for many chemical reactions and biological processes. The speaker provides examples of common weak acids and their conjugate bases, such as HF and F-, and explains how these combinations work to protect water from drastic pH changes. The concept of pKa and its relationship to Ka is introduced, with an emphasis on the practicality of using pKa in buffer calculations.
📈 Buffer Range and Polyprotic Acids
The speaker continues the discussion on buffers by explaining the buffer range, which is determined by the pKa value plus or minus one. The concept is illustrated with examples, showing how the pH changes when the ratio of acid to base in the buffer shifts. The video also introduces polyprotic acids, like carbonic acid, and their multiple pKa values, which result in multiple buffer ranges. The relevance of these buffers in biological systems, such as blood, is highlighted, emphasizing the role of carbonic acid in maintaining blood pH.
🥼 Preparing Buffer Solutions
This section focuses on the different methods of preparing buffer solutions. It explains that while the simplest way is to mix a weak acid with its conjugate base in a one-to-one ratio, there are other approaches. These include mixing a weak acid with a strong base in a two-to-one ratio or vice versa with a weak base and a strong acid. The speaker clarifies that the goal is to achieve a one-to-one ratio of weak acid to conjugate base in the final solution. The section also addresses common misconceptions about buffer preparation and emphasizes the importance of understanding the correct ratios and components.
🧬 Henderson-Hasselbalch Equation and pH Calculations
The Henderson-Hasselbalch equation is introduced as a fundamental tool for calculating the pH of buffer solutions. The speaker explains that this equation is derived from the Ka expression and is particularly useful when both the weak acid and its conjugate base are present in the solution. The section clarifies the different versions of the equation and settles on the most commonly used form for the lesson. The process of calculating pH using the Henderson-Hasselbalch equation is demonstrated, with an emphasis on the significance of the pKa value and the ratio of conjugate base to weak acid in determining the pH.
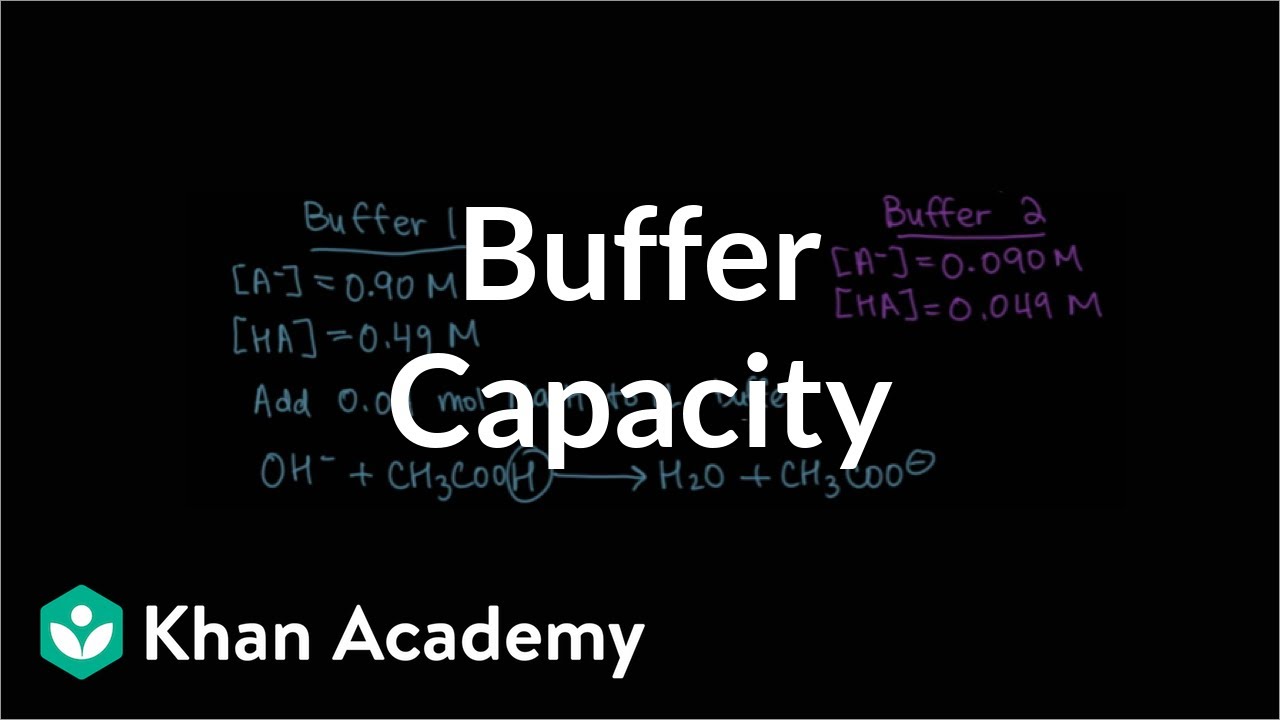
🧪 Buffer Calculations and Neutralization Reactions
The speaker provides a detailed walkthrough of how to perform pH calculations for buffer solutions, using a specific example with acetic acid and sodium acetate. The concept of significant and insignificant species in buffer solutions is discussed, and the ICE table method is deemed unnecessary for buffers. The lesson then moves on to more complex calculations involving the addition of strong acids or bases to buffer solutions. The speaker explains how to handle such scenarios, focusing on the limiting reagent concept and the changes in moles of the buffer components due to neutralization reactions.
📊 Buffers in Practice and Further Learning
In the concluding paragraph, the speaker summarizes the key points about buffer calculations and their practical applications, particularly in biochemistry. He emphasizes the importance of understanding how adding strong acids or bases to a buffer solution affects pH and the buffer's ability to resist pH changes. The speaker also provides resources for further learning, including a General Chemistry Master Course for additional practice and guidance. The lesson ends with an encouragement for viewers to support the channel and explore the available resources for continued learning and mastery of general chemistry concepts.
Mindmap
Keywords
💡Buffer solution
💡pH
💡Weak acid
💡Conjugate base
💡pKa
💡Henderson-Hasselbalch equation
💡DAT, MCAT, OAT
💡General chemistry
💡Chemical reactions
💡Living organisms
💡Salt
💡Biochemistry
Highlights
Buffers are solutions that resist changes in pH, composed of a weak acid with its conjugate base or a weak base with its conjugate acid.
The importance of buffers lies in their ability to maintain a narrow pH range, which is crucial for many chemical reactions and living organisms.
A buffer's maximum buffering capacity occurs when the ratio of weak acid to conjugate base is close to a one-to-one ratio.
The pH of a buffer solution is often close to the pKa of the weak acid or the pKb of the weak base, with a buffer range of plus or minus one around the pKa or pKb.
Buffers can be prepared by mixing a weak acid with its conjugate base in a one-to-one ratio, or by mixing a weak acid with a strong base or a weak base with a strong acid in a two-to-one ratio.
The Henderson-Hasselbalch equation is a convenient way to calculate the pH of a buffer, derived from the Ka or Kb expression.
When a strong acid or base is added to a buffer, the pH changes minimally due to the neutralization reaction that occurs, maintaining the buffer's pH within the buffer range.
The buffer range is determined by the pKa plus or minus one, which defines the pH range where the buffer can effectively resist changes.
Polyprotic acids, which have more than one acidic proton, can create multiple buffer ranges due to their different pKa values for each proton dissociation.
Carbonic acid is a diprotic acid with two pKa values, allowing it to function as a buffer in two different pH ranges, which is important in biological systems like blood.
Phosphoric acid, a polyprotic acid with three pKa values, can create three different buffer ranges, one of which is commonly used in biological systems around physiological pH.
The process of creating a buffer involves understanding the neutralization reaction between a weak acid and a strong base or a weak base and a strong acid.
When calculating the pH of a buffer after adding a strong acid or base, the key is to adjust the moles of the conjugate base and conjugate acid based on the limiting reagent.
The Henderson-Hasselbalch equation can be simplified to use moles instead of molarities when dealing with buffer solutions that involve neutralization reactions.
The concept of buffers is fundamental in general chemistry and has extensive applications in biochemistry and physiological systems.
For advanced studies in biochemistry, a deeper understanding of buffer preparation and the Henderson-Hasselbalch equation is essential for creating effective buffer solutions in the lab.
The lesson emphasizes the practical applications of buffers in maintaining pH stability in various scientific and biological contexts.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: