pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
TLDRThis video script provides an in-depth guide on calculating the pH of weak acid and base solutions, emphasizing the importance of understanding ionization and equilibrium. It introduces key concepts such as the acid dissociation constant (Ka), base dissociation constant (Kb), and their relationship with pH and pOH. The script also explains how to calculate percent ionization and offers step-by-step solutions to practice problems involving acetic acid, ammonia, ammonium chloride, and sodium fluoride, enhancing the viewer's comprehension of acid-base chemistry.
Takeaways
- 📚 The pH of a weak acid or base solution is calculated differently than that of a strong acid or base due to their incomplete dissociation in water.
- 🔄 The equilibrium of a weak acid or base reaction is represented by a double arrow, indicating a reversible reaction.
- 🍎 The Acid Dissociation Constant (Ka) and Base Dissociation Constant (Kb) are key to calculating the pH and PoH of weak acid and base solutions, respectively.
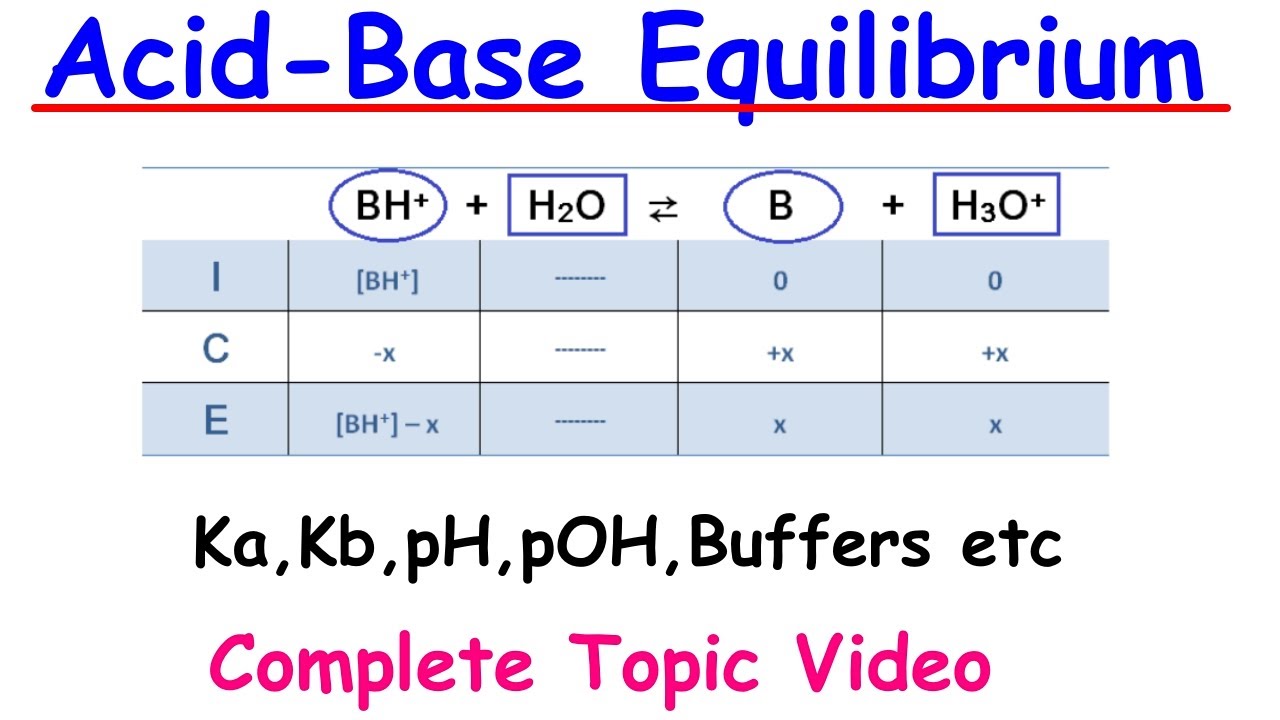
- 📈 ICE (Initial, Change, Equilibrium) tables are used to visualize and calculate the concentrations of reactants and products at equilibrium.
- 🔢 The relationship between Ka, Kb, and Kw (the auto-ionization constant of water) is given by the equation Ka * Kb = Kw at 25°C.
- 📉 The strength of an acid increases with increasing Ka values, and the stronger acid has a lower pKa value.
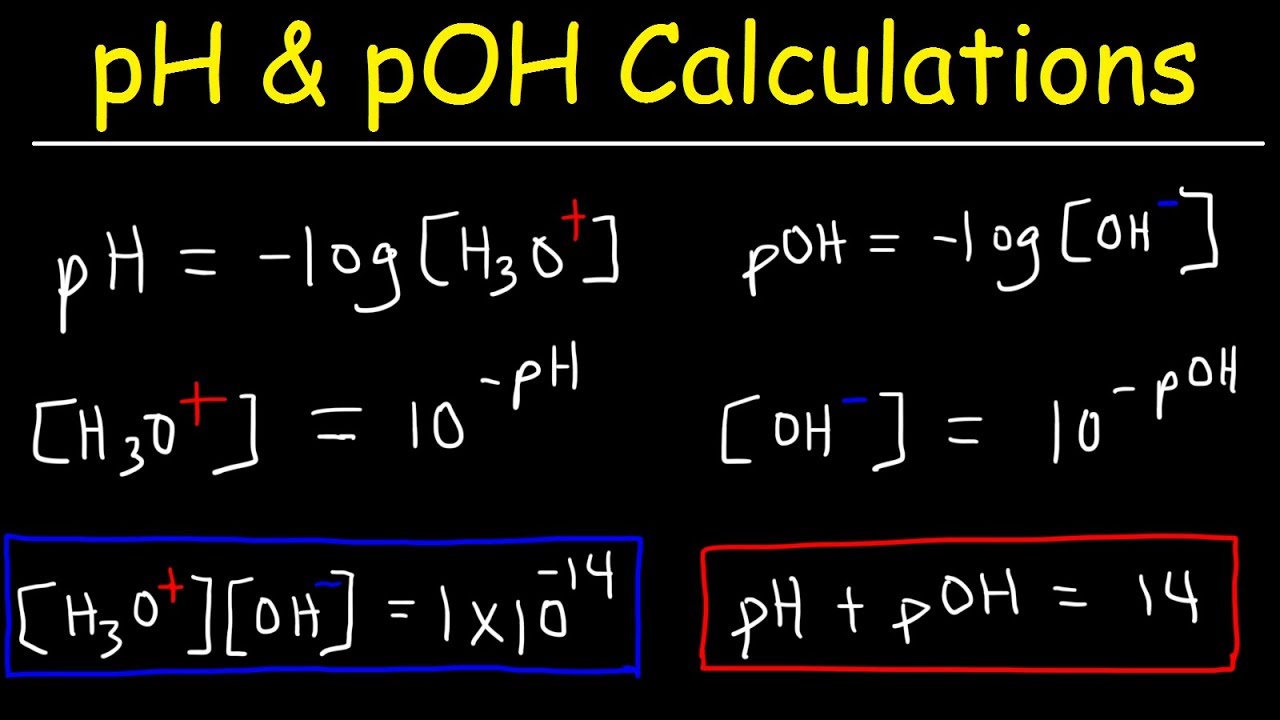
- 📊 The pH and PoH of a solution add up to 14 at 25°C, allowing the calculation of one from the other.
- 🧪 Percent ionization (also known as percent dissociation) is calculated by the formula: (x / initial acid concentration) * 100%, where x is the amount of the acid dissociated.
- 🌡️ To calculate the pH from Ka, use the formula pH = -log[H3O+], and for Kb, calculate the hydroxide ion concentration first and then find the PoH and subsequently the pH.
- 📐 The process of finding the pH of a salt solution involves recognizing the weak acid or base components and using the appropriate equilibrium constant (Ka or Kb).
Q & A
What is the main topic of the video?
-The main topic of the video is how to calculate the pH of weak acid and weak base solutions, including understanding concepts like percent ionization and using equilibrium constants such as Ka and Kb.
How does a strong acid differ from a weak acid when reacting with water?
-A strong acid, like hydrochloric acid (HCl), dissociates completely when it reacts with water, whereas a weak acid does not dissociate completely, resulting in a reversible reaction with an equilibrium established between the reactants and products.
What is the formula to calculate the pH of a solution?
-The formula to calculate the pH of a solution is pH = -log[H3O+], where [H3O+] represents the concentration of hydronium ions in the solution.
What is the relationship between Ka, Kb, and Kw?
-Ka (acid dissociation constant) times Kb (base dissociation constant) equals Kw (auto ionization constant for water), which is 1 x 10^-14 at 25 degrees Celsius. This relationship holds true at this temperature.
How is the strength of an acid related to its Ka value?
-The strength of an acid increases with increasing Ka values. A stronger acid will have a higher Ka value compared to a weaker acid.
What is the formula to calculate the percent ionization of a weak acid?
-The formula to calculate the percent ionization is (x / initial acid concentration) * 100%, where x represents the amount of the acid that has dissociated.
How do you calculate the pH of a 0.75 Molar acetic acid solution given its Ka value?
-You would set up an ICE (Initial, Change, Equilibrium) table, use the Ka value to find the change in concentration (x), and then apply the calculated x (hydronium ion concentration) to the pH formula: pH = -log[H3O+]. For acetic acid with Ka = 1.8 x 10^-5, the approximate pH would be around 2.43.
What is the pH of a 0.25 molar ammonia solution given its Kb value?
-For a 0.25 molar ammonia solution, you would use the Kb value to find the hydroxide ion concentration (x), then calculate the pOH using the formula pOH = -log[OH-], and finally find the pH by subtracting the pOH from 14. With Kb = 1.8 x 10^-5, the approximate pH would be around 11.33.
How do you find the pH of a 0.4 molar ammonium chloride solution?
-Ammonium chloride is a salt derived from a weak base (NH3), so you would first find the Ka for NH4+ using the relationship Ka = Kw / Kb. Then, set up an ICE table and use the Ka value to find the hydronium ion concentration (x), and calculate the pH using the formula pH = -log[H3O+]. The approximate pH for this solution is about 4.83.
What is the process to calculate the pH of a 1.5 molar sodium fluoride solution given the Ka of hydrofluoric acid?
-Since fluoride is the conjugate base of a weak acid (HF), you would first find the Kb for fluoride using the relationship Kb = Kw / Ka. Then, set up an ICE table and use the Kb value to find the hydroxide ion concentration (x), calculate the pOH using the formula pOH = -log[OH-], and finally find the pH by subtracting the pOH from 14. The approximate pH for this solution is about 8.66.
How can you estimate the percent ionization of a weak acid from its Ka value?
-You can estimate the percent ionization by using the formula (x / initial acid concentration) * 100%, where x is the change in concentration of the acid due to dissociation. If Ka is small, you can approximate that x is much smaller than the initial concentration, and thus, the percent ionization will be small. For example, with a Ka of 7.2 x 10^-4 and an initial concentration of 0.75, the estimated percent ionization is about 3.1%.
Outlines
📚 Introduction to Weak Acids and Bases
This paragraph introduces the concept of weak acids and bases, explaining how they differ from strong acids and bases in terms of dissociation in water. It emphasizes the importance of understanding the equilibrium constant (Ka for acids and Kb for bases) and how to use them to calculate pH and PoH. The paragraph also reviews the relationship between pH and PoH, and introduces the concept of ionization percentage, setting the foundation for further calculations and discussions in the video.
📈 Calculating pH of Weak Acid Solutions
This paragraph delves into the process of calculating the pH of a weak acid solution, using acetic acid as an example. It explains the reversible reaction of a weak acid with water and the use of an ICE (Initial, Change, Equilibrium) table to determine the concentration of hydronium ions. The paragraph outlines the steps to calculate the Ka value and apply it to find the pH, providing a clear methodology for solving such problems.
📊 Determining pH of Weak Base Solutions
This paragraph focuses on the calculation of the pH for a weak base solution, specifically using ammonia as an example. It details the reaction of a weak base with water, the use of Kb, and the construction of an ICE table to find the hydroxide ion concentration. The summary highlights the process of calculating the PoH and subsequently the pH, reinforcing the understanding of weak base behavior in solution.
🧪 pH Calculation for Salt Solutions of Weak Acids
This paragraph addresses the calculation of pH for a salt solution derived from a weak acid, using ammonium chloride as an example. It explains the identification of the weak acid (NH4+) and the use of Ka to determine the concentration of hydronium ions. The paragraph clarifies the process of finding Ka from the given Kb and applying it to calculate the pH, providing insights into the behavior of salts of weak acids in solution.
🌡️ pH of Basic Salt Solutions and Percent Ionization
This paragraph discusses the calculation of pH for a basic salt solution, using sodium fluoride and hydrofluoric acid as examples. It explains the weak base behavior of fluoride ions and the use of Kb to find the hydroxide ion concentration. The summary outlines the steps to calculate the PoH and pH, and introduces the concept of percent ionization, providing a comprehensive understanding of how basic salts can affect the pH of a solution.
📉 Estimating Percent Ionization for Weak Acids
This paragraph provides an estimation of the percent ionization for a weak acid, using hydrofluoric acid as an example. It explains the ICE table method and the calculation of Ka, highlighting the reversible reaction of the weak acid with water. The summary emphasizes the estimation process for percent ionization, offering a practical approach to understanding the degree of dissociation in weak acid solutions.
Mindmap
Keywords
💡pH
💡Weak Acid
💡Acid Dissociation Constant (Ka)
💡Percent Ionization
💡ICE Table
💡Hydronium Ion
💡Weak Base
💡Base Dissociation Constant (Kb)
💡pOH
💡Auto Ionization Constant (Kw)
💡Conjugate Acid-Base Pair
Highlights
The video discusses how to calculate the pH of weak acid and base solutions, as well as percent ionization.
Strong acids like hydrochloric acid dissociate completely, allowing for straightforward pH calculation using the formula pH = -log[H3O+].
Weak acids do not dissociate completely, and their behavior is represented by a double arrow indicating a reversible reaction at equilibrium.
The acid dissociation constant (Ka) is introduced as an equilibrium constant for weak acids, calculated as the concentration of products divided by the concentration of reactants.
The base dissociation constant (Kb) is used for weak bases, similar to Ka but for bases, and is also calculated as the concentration of products divided by the concentration of the reactant.
The relationship between Ka, Kb, and the autoionization constant of water (Kw) is given, with Ka * Kb = Kw at 25 degrees Celsius.
The concept of percent association or ionization is explained, which is calculated as the ionized concentration divided by the initial acid concentration, multiplied by 100%.
A method for calculating the pH of a weak acid solution using an ICE table and the given Ka value is demonstrated with acetic acid as an example.
The process for finding the pH of a weak base solution using Kb and an ICE table is shown, using ammonia as an example.
The calculation of the pH for a salt solution, specifically ammonium chloride, is discussed, highlighting the use of Ka for the weak acid NH4+.
The pH of a 1.5 molar sodium fluoride solution is calculated, given the Ka of hydrofluoric acid, treating fluoride as a weak base.
The impact of temperature on the values of Ka and Kb, and thus the pH calculations, is mentioned as being temperature dependent.
The relationship between acid strength, Ka values, and the pH scale is explained, with stronger acids having higher Ka values and lower pKa values.
The pKa and pKb values add up to 14 at 25 degrees Celsius, providing a check for the calculations of pH and poh.
The method for calculating the hydronium ion concentration from pH and the hydroxide ion concentration from poh is described.
The video provides a step-by-step approach to solving practice problems involving weak acids and bases, emphasizing the use of ICE tables and equilibrium constants.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: