Chapter 18: Electromagnetic Spectrum | CHM 214 | 151
TLDRThe video script delves into the intricacies of the electromagnetic spectrum, highlighting the range of visible light and its significance in analytical chemistry. It explains the relationship between wavelength, frequency, and energy, and how these properties are utilized in various spectroscopic techniques such as UV-Vis, IR, and rotational spectroscopy. The script also touches on the dangers of high-energy light, such as UV and X-rays, which can damage biomolecules. This comprehensive overview provides a foundational understanding of how light interacts with matter and its applications in scientific research.
Takeaways
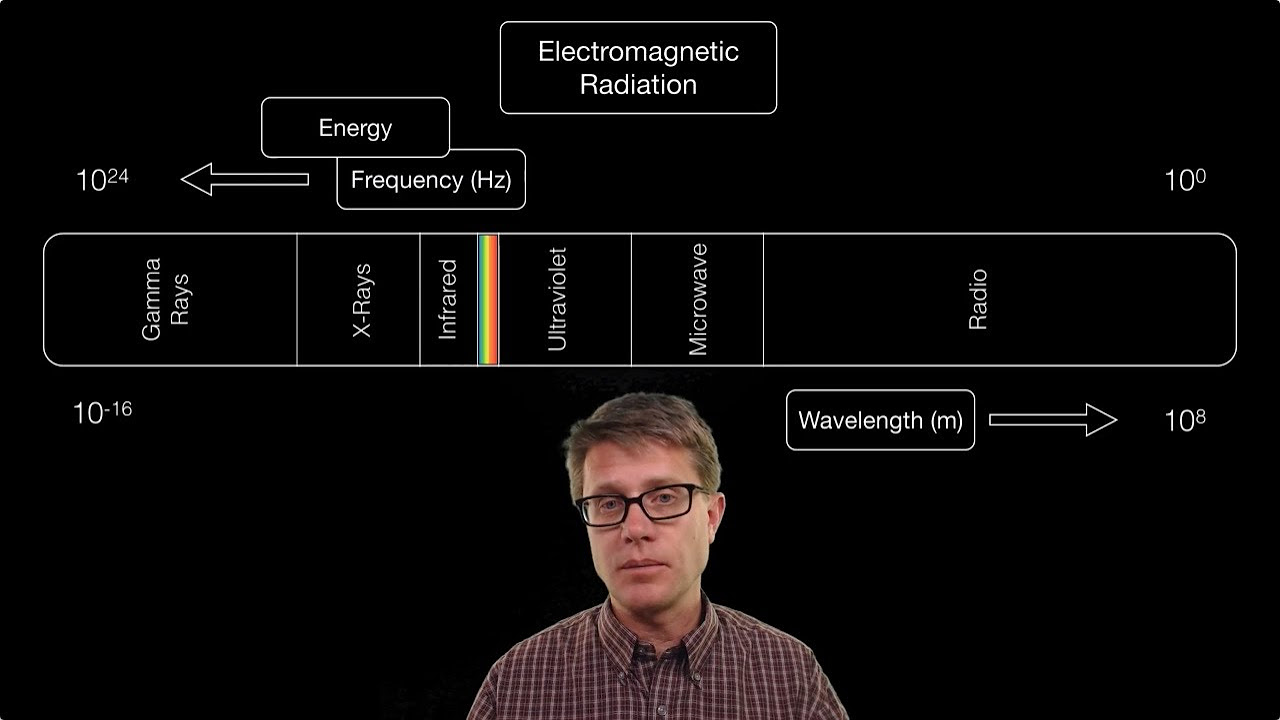
- 🌈 Visible light is a small part of the electromagnetic spectrum, which includes a range of wavelengths from radio waves to gamma rays.
- 🔬 The energy of light is inversely related to its wavelength; as wavelength increases, energy decreases, and vice versa.
- 🎨 In the visible spectrum, violet light has the highest energy while red light has the lowest.
- 🌟 UV-Vis spectroscopy is a common analytical chemistry technique that relies on the excitation of electrons within molecules or atoms.
- 🚀 As we move to lower energy levels in the spectrum, we observe molecular vibrations, which is the focus of infrared spectroscopy.
- 🔍 Infrared spectroscopy is used in organic chemistry to identify specific functional groups based on their characteristic frequencies.
- 💫 Rotational spectroscopy, though less common, is an area of active research for its potential in measuring concentrations of substances.
- 🌐 Radio waves are at the lower energy end of the spectrum, and NMR spectroscopy operates in this region, though it's not explicitly mentioned in the script.
- ⚡ Higher energy light, such as ultraviolet, X-rays, and gamma rays, can be harmful as they have enough energy to break bonds in biomolecules.
- 🏥 Precautions are necessary when using high-energy light for medical imaging, like X-rays, to protect the body from potential damage.
- 📚 Understanding the relationship between frequency, wavelength, and energy is crucial for analyzing and interpreting spectroscopic data.
Q & A
What is the range of visible light in terms of wavelength?
-Visible light is in the nanometer scale of wavelength, specifically ranging from approximately 400 to 700 nanometers.
How does the energy of light relate to its wavelength and frequency?
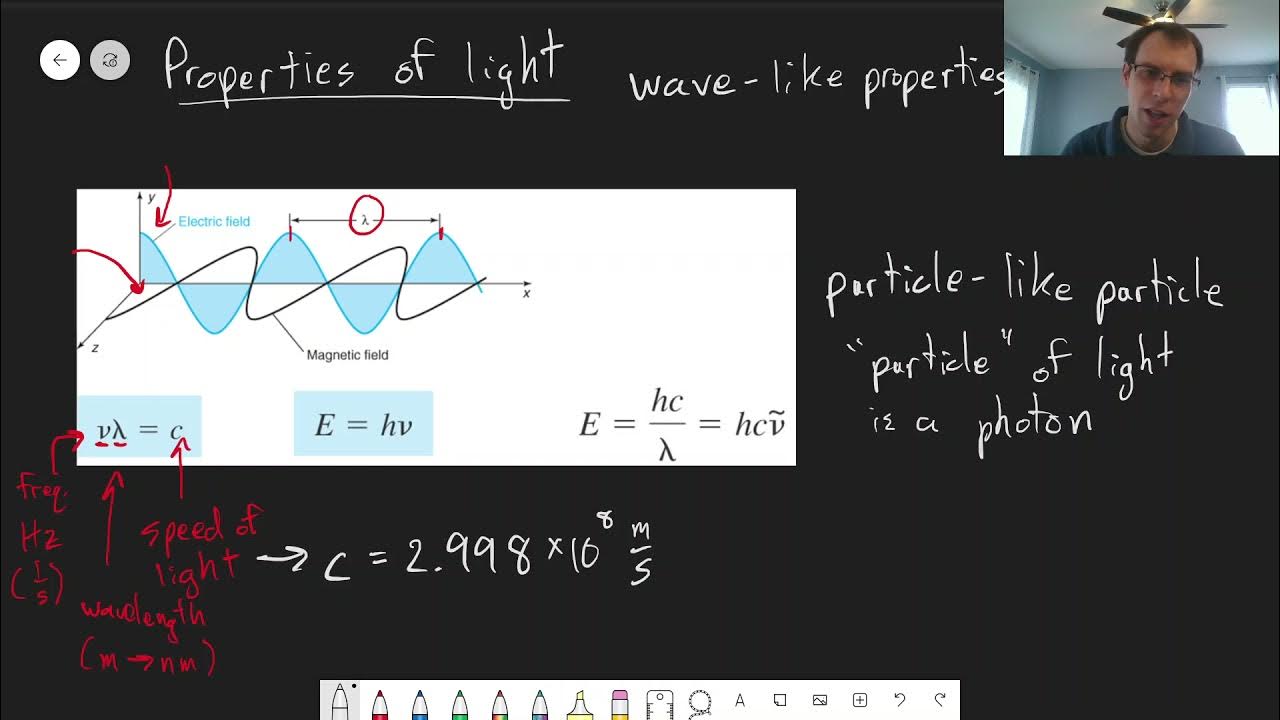
-The energy of light is inversely proportional to its wavelength and directly proportional to its frequency, as described by the equation E = h * nu, where E is energy, h is Planck's constant, and nu is the frequency.
Which part of the electromagnetic spectrum has the highest energy?
-Gamma rays have the highest energy in the electromagnetic spectrum, as they are at the far end of higher energy, shorter wavelength region.
What does UV-Vis spectroscopy focus on in terms of molecular analysis?
-UV-Vis spectroscopy focuses on the excitation of electrons within molecules or atoms, typically observing the promotion of electrons from one orbital to another.
What aspect of molecular structure does infrared (IR) spectroscopy examine?
-Infrared (IR) spectroscopy examines the vibrations of molecules, such as bond stretching, bending, or twisting.
How does rotational spectroscopy differ from other types of spectroscopy mentioned in the script?
-Rotational spectroscopy looks at the energy levels associated with molecules spinning around their axes, which is less commonly used in analytical chemistry compared to UV-Vis and IR spectroscopy.
What is the significance of specific functional groups in organic chemistry?
-Specific functional groups in organic chemistry are important because they have characteristic frequencies that can be identified using infrared spectroscopy, allowing for the characterization of molecules.
Why is it necessary to protect against higher energy light such as ultraviolet and X-rays?
-Higher energy light like ultraviolet and X-rays can be harmful as they have enough energy to break bonds within biomolecules, which can lead to skin damage or other health issues.
What is the role of NMR spectroscopy in the electromagnetic spectrum?
-Nuclear Magnetic Resonance (NMR) spectroscopy operates at the lower energy end of the electromagnetic spectrum, near the radio wave region, and is used to determine molecular structure by observing the resonance frequencies of atomic nuclei.
How can the equations relating frequency, wavelength, and energy help in understanding spectroscopy?
-These equations are fundamental in understanding the specific transitions observed in spectroscopy, as they provide a mathematical framework to relate observed spectral lines to the energy changes occurring within molecules or atoms.
What is the main application of infrared spectroscopy in analytical chemistry?
-The main application of infrared spectroscopy in analytical chemistry is to identify and characterize functional groups in organic molecules by recognizing their unique absorption bands.
Outlines
🌟 Understanding the Electromagnetic Spectrum
The paragraph introduces the concept of the electromagnetic spectrum, emphasizing the variety of light types, with a focus on visible light. It explains that visible light represents a small portion of the spectrum, characterized by its wavelength in the nanometer scale. The relationship between wavelength, frequency, and energy is discussed, highlighting that an increase in wavelength corresponds to a decrease in energy and frequency. The paragraph also outlines the high-energy end of the spectrum, starting from violet light and moving towards lower energy red light. The significance of the visible spectrum in analytical chemistry is mentioned, along with the use of UV-Vis spectroscopy to study electronic excitations in molecules and atoms. The paragraph further explores the infrared part of the spectrum, which is crucial for studying molecular vibrations and is commonly used in organic chemistry to identify specific functional groups. The discussion extends to rotational spectroscopy and its potential advantages in analytical chemistry, as well as the higher energy spectrum including ultraviolet, X-rays, and gamma rays, noting the dangers of high-energy light exposure to human health.
Mindmap
Keywords
💡Electromagnetic Spectrum
💡Visible Light
💡Wavelength
💡Energy
💡UV-Vis Spectroscopy
💡Infrared Spectroscopy
💡Rotational Spectroscopy
💡Nuclear Magnetic Resonance (NMR) Spectroscopy
💡X-Rays
💡Functional Groups
💡Analytical Chemistry
Highlights
Light comes in various forms, with visible light being the most familiar to us.
Visible light is a small part of the electromagnetic spectrum, with wavelengths measured in nanometers.
The energy of light decreases as its wavelength increases, and vice versa for frequency.
The visible spectrum ranges from high-energy violet light to low-energy red light.
UV-Vis spectroscopy is a widely used analytical chemistry technique that relies on the excitation of electrons within molecules or atoms.
Infrared spectroscopy focuses on the vibrations of molecules, such as bond stretching, bending, or twisting.
Organic chemistry often utilizes infrared spectroscopy to identify specific functional groups in molecules.
Rotational spectroscopy examines the energy levels associated with molecules spinning around their axes.
NMR spectroscopy is used as we move to lower energy levels in the electromagnetic spectrum.
Higher energy light, such as ultraviolet, can cause bonds to break in biomolecules, posing health risks.
X-rays and gamma rays have sufficiently high energy to break bonds, which is why they can be harmful to humans.
The electromagnetic spectrum is crucial for understanding transitions observed in spectroscopy.
Analytical chemistry commonly uses the visible and ultraviolet regions of the electromagnetic spectrum.
The relationship between frequency, wavelength, and energy is fundamental to the study of spectroscopy.
Research is ongoing to expand the use of rotational spectroscopy for analytical purposes, such as measuring concentrations.
The upcoming video will demonstrate calculations using equations relating frequency, wavelength, and energy.
Transcripts
Browse More Related Video
Electromagnetic Radiation

Light waves, visible and invisible

Introduction To Light | Properties of Light | Introduction to Light | properties of light | letstute

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light
Chapter 18: Properties of Light | CHM 214 | 150

Quiz Time | Basics Of Light | Physics | Science | LetsTute
5.0 / 5 (0 votes)
Thanks for rating: