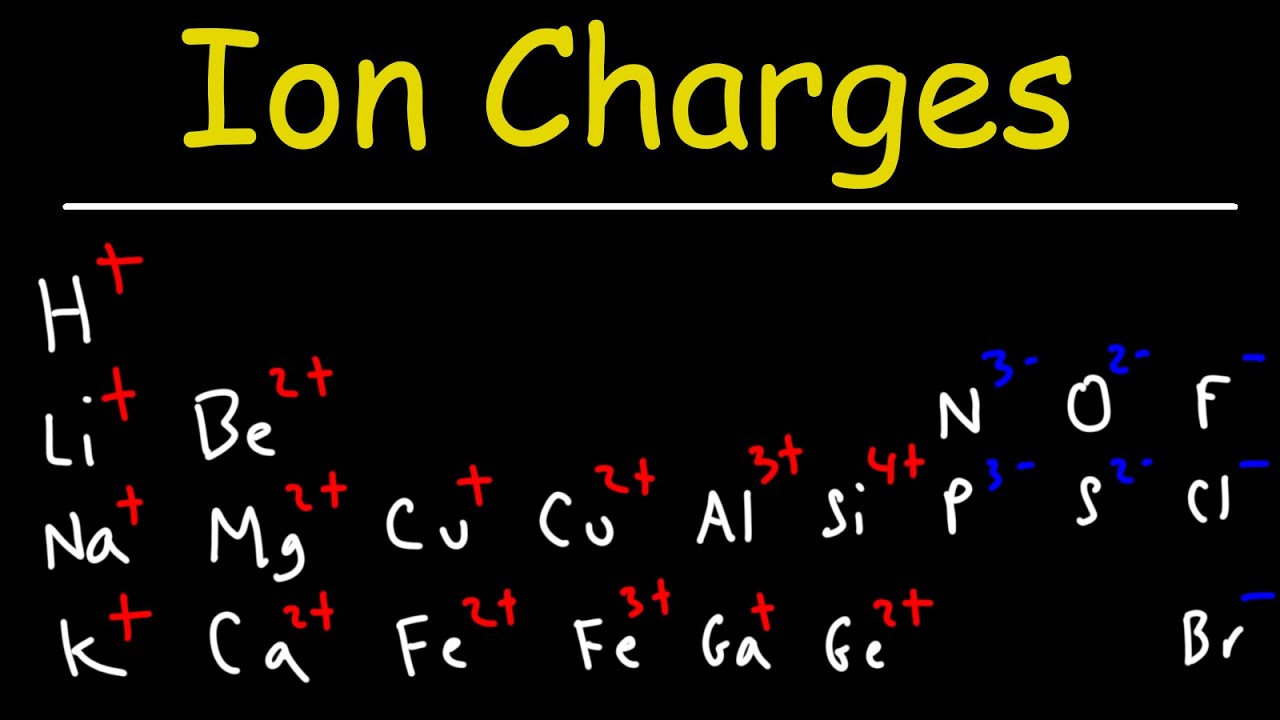Electron Configuration - Basic introduction
TLDRThe video script offers a clear and concise guide on how to write electron configurations for elements, focusing on nitrogen, aluminum, iron (Fe 2+), and the chloride ion. It explains the process of filling electron sublevels starting from 1s and progressing through energy levels, emphasizing the importance of subtracting or adding electrons based on the charge for ions. The script provides step-by-step examples, highlighting the method for transition metals and non-transition elements, ensuring a comprehensive understanding of electron configurations.
Takeaways
- 📚 The electron configuration of an element is written based on the number of electrons in the atom or ion.
- 📈 Start with the lowest energy levels (1s, 2s, 2p, etc.) and fill the sublevels according to their maximum capacities (2 for s, 6 for p, etc.).
- 👤 For neutral atoms, the number of protons equals the number of electrons, but for ions, this may differ.
- 🔢 Nitrogen has 7 electrons and its electron configuration is 1s² 2s² 2p³.
- 📊 Aluminum has 13 electrons and its electron configuration is 1s² 2s² 2p⁶ 3s² 3p¹.
- 🤖 For ions like Fe²⁺, subtract the charge value from the parent atom's electron count (26 for Fe) to get 24 electrons, then write the configuration accordingly.
- 🧩 For transition metals, write the electron configuration of the neutral atom first, then adjust for the ion's charge by removing or adding electrons from the highest energy level.
- 🌟 For non-transition metal ions, you can directly write the electron configuration based on the ion's electron count without writing the parent atom's configuration.
- 🚫 When dealing with positive ions, remove electrons from the highest energy level to match the ion's charge.
- 🔄 For negative ions, add electrons to the highest energy level to match the ion's electron count.
- 🧬 The electron configuration for the chloride ion (Cl⁻) is 1s² 2s² 2p⁶ 3s² 3p⁶, differing from chlorine's by one additional electron in the 3p sublevel.
Q & A
What is the electron configuration of nitrogen?
-The electron configuration of nitrogen, which has 7 electrons, is 1s2 2s2 2p3.
How many electrons can the s and p sublevels hold in the first and second energy levels?
-In the first energy level, the s sublevel can hold 2 electrons. In the second energy level, the s sublevel can also hold 2 electrons, and the p sublevel can hold up to 6 electrons.
What is the atomic number of aluminum and how many electrons does it have?
-Aluminum has an atomic number of 13, meaning it has 13 electrons.
Write the electron configuration for an atom of aluminum.
-The electron configuration for an atom of aluminum is 1s2 2s2 2p6 3s2 3p1.
How does the charge of an ion affect its electron configuration?
-For a positively charged ion, electrons are subtracted equal to the charge value, starting from the highest energy level. For a negatively charged ion, electrons are added to the next available sublevel.
What is the electron configuration for Fe 2+?
-The electron configuration for Fe 2+ is 1s2 2s2 2p6 3s2 3p6 4s2 or, more commonly simplified as 1s2 2s2 2p6 3s2 3p6 3d6.
How does one determine the number of electrons to add or remove for ions?
-For positively charged ions (cations), subtract the charge value from the parent atom's electron count. For negatively charged ions (anions), add the charge value to the parent atom's electron count.
What is the electron configuration of the chloride ion?
-The electron configuration of the chloride ion, which has 18 electrons, is 1s2 2s2 2p6 3s2 3p6.
Why is it important to consider the electron configuration of the parent atom when dealing with ions?
-Considering the parent atom's electron configuration helps to easily determine the number of electrons to add or remove for ions, ensuring the correct electron configuration is written for the ion.
What is the difference in writing electron configurations for transition metals versus non-transition metals?
-For transition metals, it is best to write the electron configuration of the parent atom first and then adjust for the charge. For non-transition metals, you can directly write the electron configuration based on the total number of electrons in the ion.
How does the electron configuration of an atom change when it becomes an ion?
-When an atom becomes an ion, its electron configuration changes by either gaining (for anions) or losing (for cations) electrons to achieve a stable configuration, which is reflected in the change in the electron count.
What is the significance of the electron configuration in understanding the properties of elements and their ions?
-The electron configuration is significant as it provides insight into the chemical behavior of elements and their ions, including their reactivity, bonding tendencies, and stability in different chemical environments.
Outlines
📚 Introduction to Writing Electron Configurations
This paragraph introduces the concept of writing electron configurations for elements, using Nitrogen and Aluminum as examples. It explains the process of filling electron sublevels (s, p, d, f) in order of increasing energy levels and reaching the total number of electrons for an atom. The paragraph also touches on the difference between atomic number and mass number, and how to adjust configurations for ions, specifically for the Fe 2+ ion by subtracting electrons from the highest energy level.
🔬 Electron Configurations for Ions
This paragraph delves into the specifics of writing electron configurations for ions, contrasting the processes for transition metals and non-transition metals. It uses the Chloride ion as an example to illustrate how to directly write the configuration based on the total number of electrons in the ion (18 for Chloride). The paragraph emphasizes the importance of adding or subtracting electrons based on the charge of the ion and provides guidance on how to handle configurations for both positively and negatively charged ions.
Mindmap
Keywords
💡Electron Configuration
💡Periodic Table
💡Atomic Number
💡Energy Levels
💡Sublevels
💡Ions
💡Transition Metals
💡Chloride Ion
💡Electrons
💡Orbitals
Highlights
Introduction to writing electron configurations for elements.
Explanation of nitrogen's electron configuration with 7 electrons.
Understanding the periodic table's atomic number and mass number.
Description of energy levels and sublevels: 1s, 2s, 2p, 3s, 3p, and 3d.
Example of aluminum's electron configuration with 13 electrons.
Process of writing electron configurations for ions, specifically Fe 2+.
Explanation of subtracting electrons for positive ions and adding for negative ions.
Procedure for writing electron configurations for transition metals and their ions.
Detailed method for writing the electron configuration of Fe 2+ by adjusting from the parent atom Fe.
Explanation of chlorine's electron configuration and its difference from the chloride ion.
Guidance on writing electron configurations for non-transition metal ions directly based on the ion's electron count.
Clarification on the electron configuration for the chloride ion with 18 electrons.
Emphasis on the importance of starting from the highest energy level when removing or adding electrons for ions.
Overview of the electron configuration process for main group elements and their ions.
Explanation of the electron configuration for the neutral chlorine atom versus the chloride ion.
Summary of the electron configuration rules and their application to different types of elements and ions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: