Energy Balance in Closed Systems | Thermodynamics | (Solved examples)
TLDRThis transcript delves into energy balance within closed systems, emphasizing the first law of thermodynamics. It explains how energy changes due to heat, work, or mass transfers can be calculated, focusing on internal energy changes. The discussion includes equations for finding internal energy, the significance of quality in pure substances, and how to plot pressure vs. specific volume graphs. Practical examples involving refrigerant 134a, water vapor, and motor oil illustrate the application of these concepts, providing a comprehensive understanding of energy balance in various scenarios.
Takeaways
- 📜 The energy balance in a closed system is governed by the first law of thermodynamics, where energy in minus energy out equals the change in the system's energy.
- 🔄 Energy transfer occurs through heat, work, or mass transfers and can result in changes in internal energy, kinetic energy, potential energy, etc.
- ⏱️ The energy balance equation, when dealing with rates, is expressed with respect to time, typically in watts (Joules per second).
- 🔢 To find the internal energy change, use the equation involving mass, initial internal energy, and final internal energy.
- 🌡️ When heat is transferred over a time interval, it's essential to consider heat transfer, work transfer, and the total energy change of the system.
- 💭 In cases of unknown heat and work interactions, the convention is to assume heat input and work output by the system.
- 📉 For pure substances, pressure and specific volume changes can be depicted on a graph with regions for compressed liquid, saturated liquid-vapor, and superheated vapor.
- 📊 When asked to draw a change in pressure and specific volume, start with a general graph of pressure vs. specific volume and plot the relevant points.
- 🌬️ Quality is the ratio of the mass of vapor to the total mass of the mixture and is crucial when dealing with pure substances and property tables.
- 🔧 Examples provided in the script demonstrate how to apply the concepts of energy balance to real-world scenarios involving refrigerants, water vapor, and motor oil.
- 🔄 The process of solving energy balance problems involves identifying known values, calculating unknowns using appropriate equations, and plotting changes on relevant diagrams.
Q & A
What is the basic principle of energy balance in a closed system?
-The basic principle of energy balance in a closed system is that the energy input minus the energy output equals the change in the energy of the system.
What are the different forms of energy transfer that can occur in a closed system?
-Energy transfer in a closed system can occur due to heat, work, or mass transfers.
What does the first law of thermodynamics relate to in the context of this script?
-The first law of thermodynamics is related to the concept of energy balance, specifically the change in internal energy, kinetic energy, potential energy, and more within a system.
How is internal energy change calculated in the context of this script?
-Internal energy change is calculated using the equation that involves the mass of the substance, its initial internal energy, and its final internal energy.
What is the significance of the pressure vs specific volume graph for pure substances?
-The pressure vs specific volume graph for pure substances is significant as it helps in visualizing the phase of the substance (compressed liquid, saturated liquid-vapor, superheated vapor) and understanding changes during processes.
What is the critical point in the context of the pressure vs specific volume graph?
-The critical point is the intersection of the saturated liquid and saturated vapor curves on the pressure vs specific volume graph, representing the conditions where the liquid and vapor phases of a substance coexist in equilibrium.
How is the quality of a mixture defined in thermodynamics?
-Quality is defined as the ratio of the mass of vapor to the total mass of the mixture in thermodynamics.
What are the main components of the energy balance equation for a closed system?
-The main components of the energy balance equation for a closed system are the heat transfer (Q), work transfer (W), and the change in internal energy (ΔU) of the system.
How is the direction of heat and work interactions assumed in cases where they are unknown?
-In cases where the direction of heat and work interactions are unknown, it is conventionally assumed that heat is transferred into the system (heat input) and work is done by the system (work output).
What is the significance of specific volume in calculating the mass of a refrigerant in a tank?
-Specific volume is significant in calculating the mass of a refrigerant in a tank because it allows us to determine the amount of substance present based on the known volume of the tank and the properties of the refrigerant at given conditions.
How does the energy balance equation account for changes in internal energy due to heat addition?
-The energy balance equation accounts for changes in internal energy due to heat addition by considering the difference between the initial and final internal energies of the system, which is equal to the heat added to the system.
Outlines
🔋 Energy Balance in Closed Systems
This paragraph introduces the concept of energy balance within closed systems, emphasizing the first law of thermodynamics. It explains how energy can enter and exit a system through heat, work, or mass transfers, leading to changes in internal energy, kinetic energy, or potential energy. The focus is on internal energy change, and the equation for energy balance over time is presented. The paragraph also touches on the assumptions made when heat and work directions are unknown, suggesting that heat is typically assumed to enter the system and work is done by the system. The importance of understanding pressure and specific volume changes, especially for pure substances, is highlighted, and the general pressure vs specific volume graph is described. The paragraph concludes with a mention of the importance of understanding quality, property tables, and their application in thermodynamics, setting the stage for examples to illustrate the concepts discussed.
📈 Calculating Internal Energy Changes
This paragraph delves into the specifics of calculating internal energy changes by identifying the initial and final internal energies (u1 and u2). It outlines the process of using equations and tables to determine these values, taking into account the specific volume, which remains constant between initial and final stages. The paragraph explains how to handle situations where the refrigerant becomes a superheated vapor, requiring the use of superheated vapor tables. It also describes the steps to draw a pressure vs specific volume diagram, providing a method for plotting the initial and final states of a substance. The paragraph then presents a problem involving a mixture of water and vapor, guiding through the process of finding the mass, specific volumes, and internal energies at different temperatures to calculate the energy transferred into the system.
🌡️ Energy Transfer in Systems with Stirring Devices
The final paragraph discusses a scenario where a stirring device is used within a container of motor oil, and both heat and shaft power are supplied to the system. It explains how to calculate the specific energy increase by dividing the total energy input by the mass of the oil. The paragraph emphasizes that the energy balance equation remains the same, regardless of the presence of additional energy inputs like shaft power. The solution involves identifying the energy inputs (heat and shaft power), applying the energy balance equation, and then dividing the result by the mass to find the specific energy increase. The paragraph concludes by summarizing the types of problems related to energy balance and hints at the next video's content, which will cover specific heats and their impact on energy balance questions.
Mindmap
Keywords
💡Energy Balance
💡Closed System
💡Internal Energy Change
💡Heat Transfer
💡Work Transfer
💡Mass Transfers
💡Specific Volume
💡Quality
💡Property Tables
💡Saturated Liquid-Vapor Region
💡Pressure vs. Specific Volume Diagram
Highlights
The energy balance in closed systems is based on the first law of thermodynamics, which states that the energy in minus the energy out equals the change in the system's energy.
Energy transfer can occur through heat, work, or mass transfers, affecting the system's internal energy, kinetic energy, potential energy, and more.
When dealing with energy transfer rates, such as in watts (Joules per second), the energy balance equation can be expressed with respect to time rate.
To find the internal energy change, one must consider the mass, initial internal energy, and final internal energy of the system.
In situations with unknown heat and work interactions, it is conventional to assume heat input and work output, with negative results indicating the opposite.
For pure substances, pressure and specific volume relationships can be plotted on a graph, with regions for compressed liquid, saturated liquid-vapor, and superheated vapor.
Quality is the ratio of the mass of vapor to the total mass of the mixture and is crucial for understanding phase changes in energy balance calculations.
Examples provided in the transcript demonstrate how to apply energy balance principles to real-world scenarios involving refrigerants, water vapor, and motor oil.
The mass of a refrigerant in a tank can be calculated using the tank's volume and the specific volume of the refrigerant at a given pressure and quality.
Heat transfer and work done can be calculated for a closed system by considering the energy input and output over a certain time interval.
A pressure vs specific volume diagram is essential for visualizing phase changes and energy transfers in pure substances.
The specific volume and internal energy values for different states of a substance can be found using property tables and used in energy balance calculations.
The concept of quality and its changes with temperature are important for accurately modeling energy transfers in mixtures.
In a scenario with a stirring device, the energy input from both heat and shaft power contributes to the system's energy balance.
The specific energy increase in a system can be calculated by dividing the total energy change by the mass of the substance.
Understanding energy balance is crucial for solving problems in thermodynamics and engineering applications.
The next video will cover specific heats and their impact on energy balance calculations, further enhancing the understanding of thermodynamic systems.
Transcripts
Browse More Related Video

Thermochemistry Equations & Formulas - Lecture Review & Practice Problems
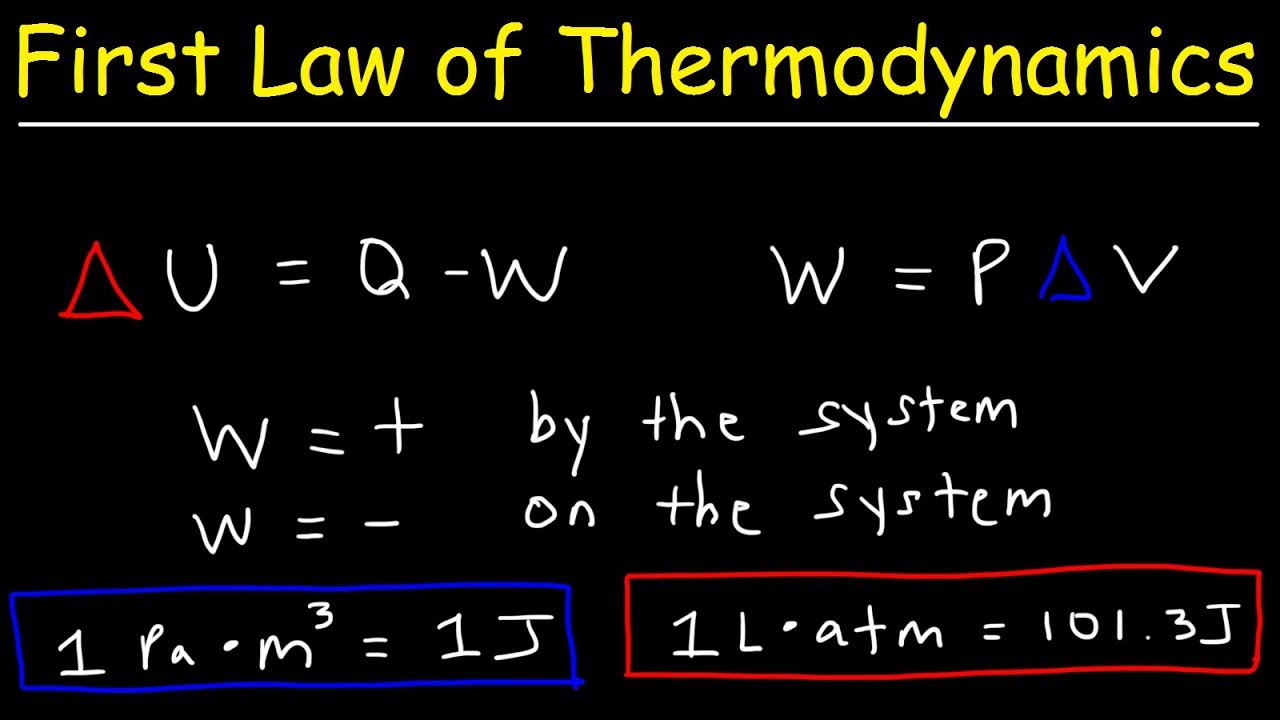
First Law of Thermodynamics, Basic Introduction, Physics Problems

First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry

Enthalpy | Thermodynamics
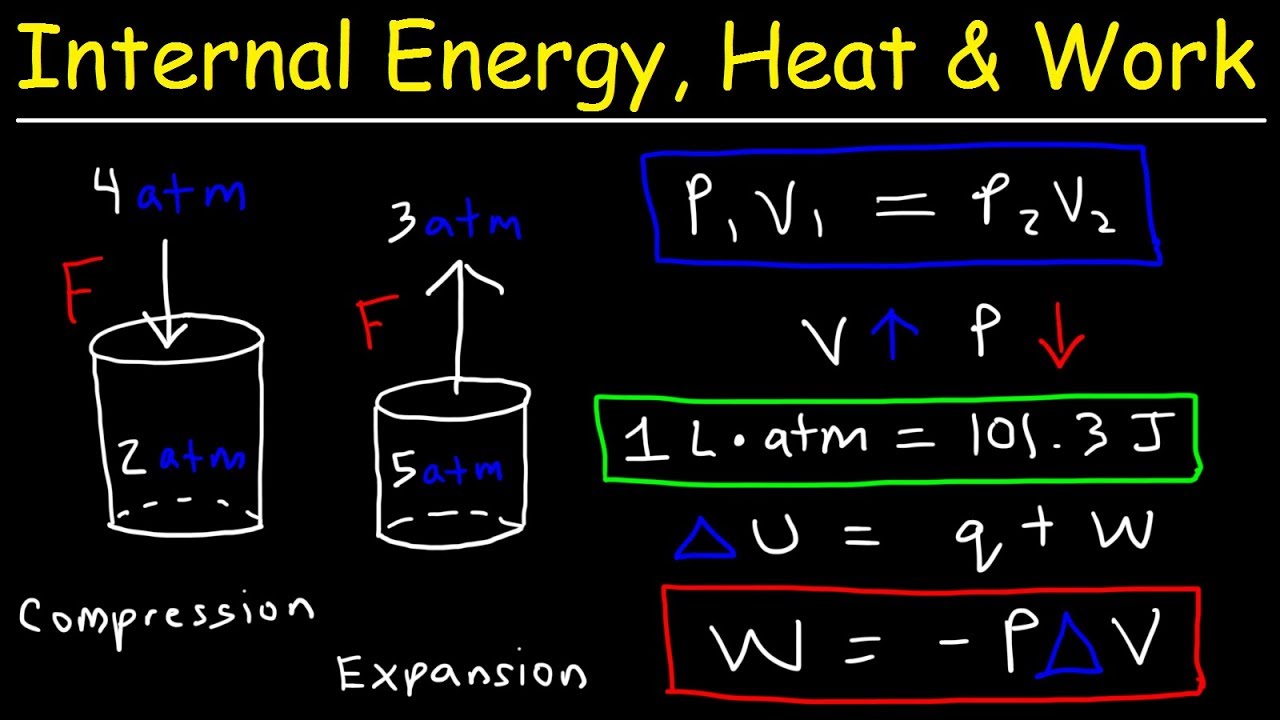
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: