Reading Skeletal Line Structures (Organic Chemistry), Parts 2 & 3
TLDRThis educational video guides viewers on how to read and interpret skeletal line structures in chemistry, focusing on determining the number of hydrogen atoms in various compounds like alkanes, alkenes, and alkynes. It also explains the significance of wedge and dash bonds in representing three-dimensional molecular orientations.
Takeaways
- 🔍 The video provides a tutorial on how to read skeletal line structures and determine the chemistry of molecules.
- 📝 The process involves counting carbon and hydrogen atoms in a molecule to deduce its chemical formula.
- 🔢 The script explains that for a molecule like C9, there are 10 possible hydrogen atoms, considering the double bond at the end.
- 💧 It clarifies that the presence of a double bond at the end of a section does not change the count of hydrogen atoms.
- 🌐 The video illustrates how to count hydrogen atoms in a five-carbon ring with a chlorine atom at the end, resulting in the formula C5H7Cl.
- 🚫 Alkanes are defined as substances without double or triple bonds, consisting only of carbon and hydrogen.
- 🔗 Alkenes contain a double bond, and alkynes contain a triple bond, which are key to understanding the structure of the molecule.
- 🔑 The script uses an example of an alkyne with a triple bond to demonstrate the counting of carbon and hydrogen atoms, resulting in C7H12.
- 📈 The video includes more complex examples, such as a 14-carbon molecule with nitrogen and oxygen atoms, showing the process of counting all atoms.
- 📚 It explains the concept of stereochemistry with the example of methane, using wedge and dash bonds to represent three-dimensional orientation.
- 👓 The final takeaway is an understanding that wedge and dash bonds in molecular structures represent the spatial arrangement of atoms in 3D space.
Q & A
What is the significance of counting hydrogen atoms in skeletal line structures?
-Counting hydrogen atoms in skeletal line structures helps determine the complete molecular formula of a compound, ensuring that all atoms are accounted for and the structure is accurately represented.
How does the presence of a double bond at the end of a carbon chain affect the number of hydrogen atoms?
-A double bond at the end of a carbon chain occupies two bond sites, leaving only one available spot for a hydrogen atom on the terminal carbon, as opposed to the usual two spots available in a single bond.
What is the molecular formula for a compound with a five-carbon ring and a chlorine atom at the end?
-The molecular formula for such a compound is C5H7Cl, as each carbon atom in the ring can have one hydrogen atom, and the chlorine atom does not affect the hydrogen count.
What are the characteristics of alkanes, alkenes, and alkynes?
-Alkanes are hydrocarbons with only single bonds between carbon atoms, alkenes have at least one double bond, and alkynes have at least one triple bond.
How does the presence of a triple bond affect the hydrogen count in a molecule?
-In a molecule with a triple bond, the carbon atoms involved in the triple bond cannot have any hydrogen atoms attached, as they are already fully bonded with four other atoms (three in the triple bond and one in the single bond to the adjacent carbon).
What is the molecular formula for a compound with a seven-carbon chain and a triple bond?
-The molecular formula for such a compound is C7H12, as each carbon atom can have a maximum of two hydrogen atoms, except for the two carbons involved in the triple bond.
How many hydrogen atoms are in a compound with a 14-carbon chain and two oxygen atoms?
-The compound would have 19 hydrogen atoms, as each carbon atom can have a maximum of two hydrogen atoms, except for the carbons involved in the oxygen bonds.
What do wedge and dash bonds represent in a molecular structure?
-Wedge and dash bonds represent single bonds in a three-dimensional space, indicating the orientation of the molecule and suggesting parts of the molecule that are coming out of the screen or going into the page.
Why is methane represented as a tetrahedral molecule despite its simple two-dimensional representation?
-Methane is a tetrahedral molecule because the carbon atom is bonded to four hydrogen atoms, which are arranged symmetrically around the carbon in three-dimensional space, forming a tetrahedral shape.
How can the presence of non-carbon and non-hydrogen atoms affect the molecular formula of a compound?
-Non-carbon and non-hydrogen atoms, such as nitrogen or oxygen, are included in the molecular formula and affect the overall composition of the compound, indicating the presence of functional groups or other elements.
Outlines
🔬 Understanding Skeletal Line Structures in Chemistry
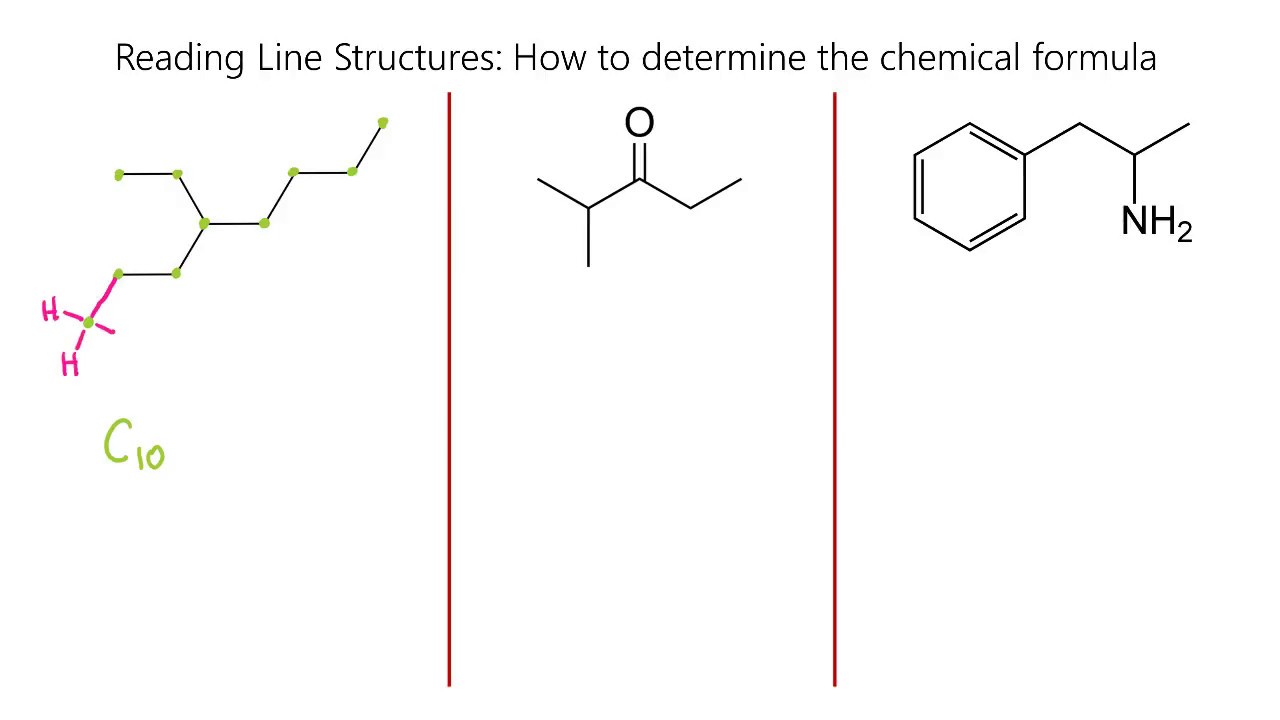
This second video on reading skeletal line structures in chemistry dives into more examples. It starts by explaining the carbon ring structure, emphasizing the counting of hydrogen atoms based on bonding patterns. A detailed example involving a carbon ring with a terminal double bond illustrates the process. The video also covers the inclusion of chlorine in a carbon ring structure, leading to the molecular formula C5H7Cl. The differentiation between alkanes, alkenes, and alkynes is explained, highlighting the characteristics of each. A specific example of an alkyne with a triple bond is discussed, including the method for determining the number of hydrogen atoms. The video concludes with a complex example involving multiple elements and bonds, resulting in the molecular formula C14H19NO2.
🧪 Advanced Molecular Structure and Stereochemistry
The final example in the video deals with a more complex molecular structure, resulting in the formula C21H26ClNO. The concept of wedge and dash bonds is introduced, explaining their significance in representing three-dimensional molecular orientation. The video clarifies that these bonds indicate the spatial arrangement of atoms, using methane as an example to illustrate tetrahedral geometry. The methane structure is shown in both two-dimensional and three-dimensional forms, demonstrating how wedge and dash bonds convey depth. The video wraps up by reinforcing the idea that these bonds provide additional information about molecular structure without changing the basic bond type.
Mindmap
Keywords
💡Skeletal line structures
💡Chemistry
💡Hydrogens
💡Double bond
💡Alkane
💡Alkene
💡Alkyne
💡Molecular formula
💡Stereochemistry
💡Wedge bonds
💡Tetrahedral
Highlights
Introduction to the process of reading skeletal line structures and determining chemistry.
Explanation of counting hydrogen atoms in a C9 structure with a double bond at the end.
Clarification on the maximum number of hydrogen bonds possible for a terminal carbon with a double bond.
Demonstration of counting hydrogen atoms in a C5 ring with a chlorine atom at the end.
Introduction to alkanes, alkenes, and alkynes, and their respective bond characteristics.
Analysis of a C7 alkyne structure and the method to determine its complete formula.
Explanation of why carbons in a triple bond cannot have hydrogen atoms attached.
Presentation of a complex C14 structure with nitrogen and oxygen atoms.
Method to count hydrogen atoms in a complex C14 molecule with multiple heteroatoms.
Introduction to C21 structure with unique bonds and a detailed count of its hydrogen atoms.
Final component identification of the C21 compound involving chlorine, nitrogen, and oxygen.
Discussion on the representation of three-dimensional space in chemical structures with wedge and dash bonds.
Explanation of the significance of wedge and dash bonds in indicating the orientation of a molecule.
Illustration of methane's tetrahedral structure to demonstrate the concept of stereochemistry.
Insight into the three-dimensional representation of methane with wedge and dash bonds.
Conclusion summarizing the importance of understanding skeletal line structures for chemistry.
Transcripts
Browse More Related Video

How to Draw Skeletal Structure or Bond-Line Notation for Organic Molecules
Reading Skeletal Line Structures (Organic Chemistry), Part 1

Organic Chemistry Drawing Structures - Bond Line, Skeletal, and Condensed Structural Formulas

16.1 Hydrocarbons | High School Chemistry

How Many Carbons And Hydrogens Are In These Compounds (Organic Chemistry)

Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]
5.0 / 5 (0 votes)
Thanks for rating: