AP® Chemistry Kinetics Questions Free Response
TLDRThis video script guides viewers through a kinetics problem similar to those found on the AP Chemistry exam. It explains how to determine reaction order from graphs, derives a rate law for a zero-order reaction with respect to CO, calculates the rate constant k using the initial reaction rate and NO2 concentration, and discusses the consistency of a proposed reaction mechanism with the rate law. Finally, it covers how to sketch a potential energy diagram for a spontaneous reaction, emphasizing the importance of activation energy and the rate-determining step.
Takeaways
- 📈 The video script discusses a kinetics problem similar to those found on the AP Chemistry exam, emphasizing the importance of graph interpretation for determining reaction order.
- 📚 The script is part of an AP Chemistry mini-test available for download on the instructor's website to aid in exam preparation.
- ✍️ For Part A, the script explains how to correlate graphs of time versus reactant concentration, ln[reactant], or 1/reactant with reaction orders, identifying a linear 1/[NO2] graph as indicative of a second-order reaction.
- 🔍 In Part B, the script instructs on writing a rate law, given that the reaction is second-order with respect to NO2 and zero-order with respect to CO, resulting in the rate law: rate = k[NO2]^2.
- 🧭 For Part C, the script details how to calculate the rate constant 'k' using the initial rate of reaction and the initial concentration of NO2 from the provided graphs, leading to a value of 0.35 L/mole·s.
- 🔄 Part D explores reaction mechanisms, explaining that the slow step in a mechanism is the rate-determining step and must include all species in the rate law, confirming the proposed mechanism's consistency with the determined rate law.
- 📉 In Part E, the script describes how to sketch a relative potential energy diagram for a spontaneous reaction, emphasizing the lower energy of products compared to reactants and the significance of activation energy barriers.
- 📝 The script highlights the importance of understanding basic concepts for the AP exam, such as reaction order determination from graphs, rate law formulation, rate constant calculation, and reaction mechanism analysis.
- 📉 The potential energy diagram should depict a negative change in Gibbs free energy (ΔG), indicating a spontaneous reaction, with the slow step having a higher activation energy barrier than the fast step.
- 📑 The script provides a step-by-step guide through a typical AP Chemistry kinetics problem, from interpreting graphs to analyzing reaction mechanisms and sketching energy diagrams.
- 🌟 The instructor encourages students to utilize the provided resources and wishes them luck on the upcoming AP Chemistry exam.
Q & A
What is the main purpose of the video script?
-The main purpose of the video script is to guide students through a kinetics problem similar to those found in the free response section of the AP Chemistry exam, providing strategies for understanding and solving such problems.
What are the three types of graphs typically used to determine the order of a reaction in kinetics?
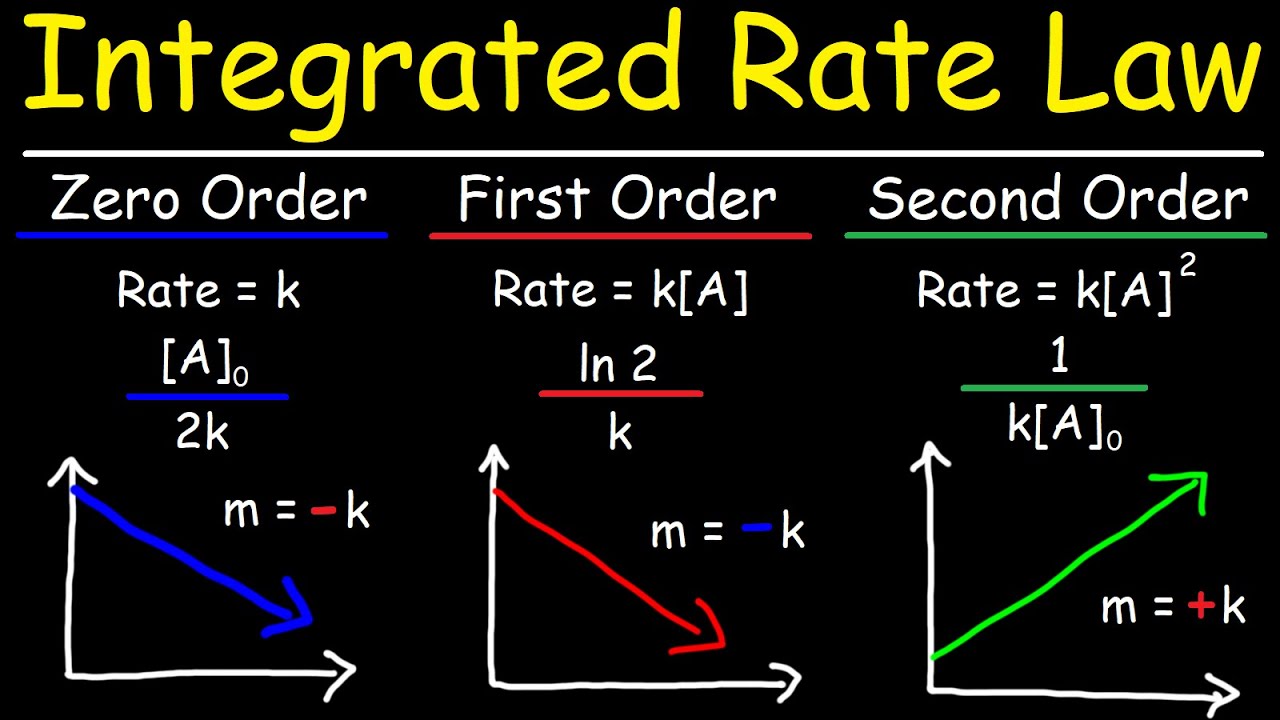
-The three types of graphs are: time versus the concentration of the reactant (A), time versus the natural log of the reactant (ln[A]), and time versus the inverse of the reactant (1/A)).
How can you determine the order of a reaction from the provided graphs?
-The order of the reaction is determined by identifying which of the three graphs is linear. A linear graph of concentration indicates a zero-order reaction, a linear ln[A] graph indicates a first-order reaction, and a linear 1/A graph indicates a second-order reaction.
What is the rate law equation and how is it used in the script?
-The rate law equation is rate = k * [A]^m * [B]^n, where k is the rate constant, [A] and [B] are the concentrations of the reactants, and m and n are the reaction orders with respect to A and B, respectively. In the script, it is used to write the rate law for a reaction given the reaction orders determined from the graphs.
Why does the CO concentration not appear in the rate law derived in part B of the script?
-The CO concentration does not appear in the rate law because the reaction is zero-order with respect to CO, meaning its concentration does not affect the reaction rate. Any term raised to the zero power equals one, so it effectively drops out of the equation.
How is the rate constant k determined in part C of the script?
-The rate constant k is determined by using the initial rate of the reaction and the initial concentration of NO2. The formula k = rate / [NO2]^2 is rearranged and solved for k using the given values.
What is the significance of the slow step in a reaction mechanism?
-The slow step is significant because it is the rate-determining step, meaning it dictates the overall rate of the reaction. It is the step that takes the longest to complete and thus limits how fast the reaction can proceed.
Why must the rate-determining step include all species in the rate law?
-The rate-determining step must include all species in the rate law because it is the step that determines the overall rate of the reaction. If any reactant is not involved in this step, it would not affect the rate, which contradicts its presence in the rate law.
How does the molecularity of a step relate to the reaction order in the rate law?
-The molecularity of a step, which refers to the number of molecules involved in the reaction, directly relates to the reaction order in the rate law. For example, a bimolecular step with respect to NO2 would result in a second-order reaction with respect to NO2.
What does a potential energy diagram represent and how is it used in part E of the script?
-A potential energy diagram represents the energy changes during a reaction, with reaction progress on the x-axis and energy on the y-axis. In part E, it is used to illustrate that the reaction is spontaneous by showing that the reactants have a higher energy (Gibbs free energy, ΔG) than the products.
What is the importance of activation energy in the context of potential energy diagrams?
-Activation energy is the minimum energy required to start the reaction. In potential energy diagrams, it is represented by energy 'bumps' or barriers that the reaction must overcome. The height and order of these bumps indicate the relative rates of the reaction steps, with the highest bump corresponding to the slow, rate-determining step.
Outlines
🔍 Analyzing Kinetics Graphs to Determine Reaction Order
This paragraph introduces an AP Chemistry exam-style kinetics problem, focusing on interpreting graphs to deduce the reaction order with respect to NO2. The speaker explains that typically, graphs show time on the x-axis and reactant concentration on the y-axis in one of three forms: linear concentration, natural log of concentration, or the inverse of concentration. The key to solving such problems is identifying which of these graphs is linear, as this indicates the reaction order. For instance, a linear concentration graph suggests a zero-order reaction, a linear ln[A] graph indicates a first-order reaction, and a linear 1/A graph points to a second-order reaction. The provided graphs show that the reaction in question is second order with respect to NO2, as the time versus 1/NO2 graph is linear.
📚 Writing the Rate Law for a Hypothetical Reaction
The speaker proceeds to explain how to write a rate law for a reaction, assuming it is zero order with respect to CO. The standard rate law formula is presented as rate = k × [reactant]^order. Given that the reaction is second order with respect to NO2 from the previous analysis, and zero order with respect to CO, the rate law simplifies to rate = k × [NO2]^2, with CO's concentration not influencing the rate due to its zero-order status. This step is crucial for understanding how the rate of a reaction is mathematically expressed in relation to the concentrations of the reactants involved.
🧭 Calculating the Rate Constant from Given Data
The paragraph discusses how to calculate the rate constant 'k' using the initial rate of the reaction and the initial concentration of NO2. The rate law derived in the previous section is rearranged to solve for k, resulting in k = rate / [NO2]^2. The initial rate is provided in the problem statement, and the initial concentration of NO2 is extracted from the kinetics graphs at time zero. The speaker emphasizes the importance of squaring the concentration value when performing the calculation, which is a common mistake among students. The calculated rate constant is found to be 0.35 L/mol·s, and the speaker notes the importance of the value over the unit when grading the AP exam.
🔧 Evaluating a Reaction Mechanism Against the Rate Law
This section delves into reaction mechanisms, specifically whether a proposed two-step mechanism aligns with the previously determined rate law. The speaker outlines key concepts about reaction mechanisms, such as the rate-determining step being the slowest and thus dictating the overall reaction rate. It is explained that this slow step must include all species present in the rate law, and the molecularity of the step is discussed, indicating how many molecules of a reactant are involved in the slow step. The provided mechanism is deemed consistent with the rate law, as the slow step only involves NO2, which is second order, aligning with the bimolecular nature of the step.
📊 Sketching a Potential Energy Diagram for a Spontaneous Reaction
The final paragraph addresses how to sketch a potential energy diagram for a spontaneous reaction, with a focus on the concepts of Gibbs free energy (delta G) and activation energy. The speaker explains that the diagram should show the reaction progress on the x-axis and energy on the y-axis, with the reactants at a higher energy level than the products due to the negative delta G of a spontaneous reaction. Activation energy is depicted as energy 'bumps', with the rate-determining step having the higher bump. The diagram should reflect that the slow step comes first, followed by the fast step with a lower activation energy bump. The speaker assures that as long as the diagram is conceptually accurate and follows these guidelines, it should meet the AP exam's expectations.
Mindmap
Keywords
💡Kinetics
💡AP Chemistry Exam
💡Reaction Order
💡Rate Law
💡Graphs
💡Rate Constant (k)
💡Potential Energy Diagram
💡Spontaneous Reaction
💡Activation Energy
💡Molecularity
💡Mechanism
Highlights
Introduction to a kinetics problem similar to those on the AP Chemistry exam.
Explanation of how to interpret graphs to determine the reaction order with respect to NO2.
Identification of the linear graph of time versus 1/NO2 as indicative of a second-order reaction.
Writing a rate law for a reaction assuming it's zero order with respect to CO.
Understanding that the reaction order of NO2 is second order, based on the rate law.
The concept that the rate law does not include CO due to its zero-order status.
Determination of the rate constant k using the initial rate of reaction and initial concentration of NO2.
Explanation of the importance of squaring the concentration of NO2 when calculating k.
Discussion on the units of the rate constant k and their dependence on reactants and their order.
Analysis of a proposed two-step reaction mechanism and its consistency with the determined rate law.
The slow step in a reaction mechanism is the rate-determining step due to its longer completion time.
The rate-determining step must include all species present in the rate law.
Zero-order reactions suggest that the reactant is not involved in the rate-determining step.
The molecularity of the slow step is bimolecular with respect to NO2, aligning with the second-order rate.
Sketching a relative potential energy diagram for a spontaneous reaction using Gibbs free energy (ΔG).
The importance of depicting the change in energy (ΔG) and activation energy in energy diagrams.
Guidelines for drawing a conceptual energy diagram that reflects the spontaneity of the reaction.
Final advice on tackling kinetics questions on the AP Chemistry exam and resources available on the instructor's website.
Transcripts
Browse More Related Video

2 | FRQ (Short) | Practice Sessions | AP Chemistry
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics

AP Chem - Full kinetics review guide

12.32 | Describe how graphical methods can be used to determine the order of a reaction and its rate

12.74 | Experiments were conducted to study the rate of the reaction represented by this equation

AP Chem Unit 5 Review - Kinetics in 10 Minutes!
5.0 / 5 (0 votes)
Thanks for rating: