Nomenclature of Polycyclic Compounds: Naphthalene, Biphenyl, Anthracene, Spiro, Bicyclo
TLDRThis educational script discusses the nomenclature of polycyclic compounds, focusing on aromatic structures like naphthalene and anthracene, as well as aliphatic ones like Spiro and bicyclo compounds. It uses ChemDoodle to demonstrate the naming process, emphasizing the importance of identifying the longest chain, numbering to give substituents the lowest locants, and understanding the spatial relationships between substituents. The script aims to clarify complex nomenclature rules through interactive examples, providing a deeper understanding of chemical naming conventions.
Takeaways
- 📝 The script covers the nomenclature of polycyclic structures, particularly focusing on aromatic and aliphatic compounds.
- 🔍 It uses ChemDoodle to explore nomenclature rules interactively, allowing for the visualization of structural changes and their impact on naming.
- 🔑 The basic principle of alkane nomenclature is reviewed, emphasizing finding the longest carbon chain and numbering from the end that gives substituents the lowest possible numbers.
- 🌐 The script explains the nomenclature of naphthalene, a polycyclic aromatic hydrocarbon with two fused benzene rings, and how to name derivatives with substituents.
- 🔄 The importance of considering molecule rotation to identify equivalent positions for substituents is highlighted, as it affects the numbering and naming of compounds.
- 🔢 The numbering scheme for polycyclic compounds is discussed, showing how to assign the lowest set of locants to substituents for proper naming.
- 🔗 The script introduces the concept of 'biphenyl' and differentiates it from 'naphthalene' based on the way the benzene rings are connected.
- 💧 The 'Spiro' prefix is used for compounds where rings are connected at a single point, and the nomenclature involves counting carbons in each ring separately.
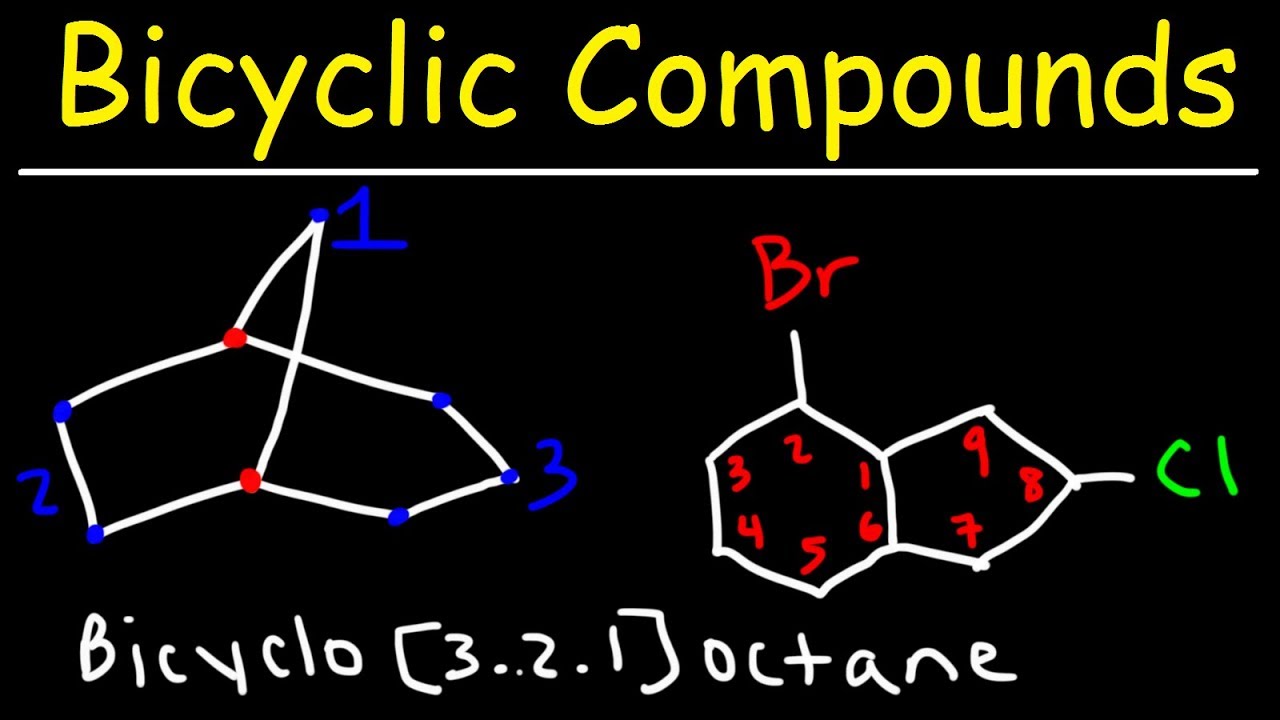
- 🔬 The 'bicyclo' nomenclature is explained for bridged ring systems, where the numbers represent the size of the rings and the total number of carbons in the structure.
- 📚 The script demonstrates how to navigate complex nomenclature by adding substituents to various positions on polycyclic compounds and observing the changes in names.
- 🚀 The interactive approach using ChemDoodle is promoted as an educational tool for better understanding and practicing the IUPAC nomenclature of polycyclic compounds.
Q & A
What is the first step in naming an alkane according to the script?
-The first step is to find the longest carbon chain in the alkane.
Why is it incorrect to consider a 5-carbon chain with an isopropyl group as the main chain in alkane nomenclature?
-It is incorrect because the longest chain should be considered, which in this case would be a 6-carbon chain, making it a hexane.
What does the term 'naphthalene' refer to in the context of polycyclic aromatic hydrocarbons?
-Naphthalene refers to a molecule consisting of two fused benzene rings.
How does the position of a substituent on a naphthalene affect its name?
-The position of a substituent determines the numerical prefix in the name, such as '1-methylnaphthalene' or '2-methylnaphthalene'.
What is the significance of numbering from the side that gives the substituents occurring soonest in alkane nomenclature?
-Numbering from the side that gives the substituents occurring soonest ensures that the lowest possible numbers are assigned to the substituents, following IUPAC nomenclature rules.
What is the term used to describe a compound with two rings sharing a single carbon atom?
-The term used is 'spiro' to describe such a compound.
How does the script explain the numbering scheme for a spiro compound?
-The numbering scheme for a spiro compound starts from the smaller ring and proceeds to the larger ring, not considering the carbon that connects the two rings.
What is the difference between 'biphenyl' and 'naphthalene' in terms of ring fusion?
-In 'biphenyl', the two benzene rings are connected by a single sigma bond, whereas in 'naphthalene', the two benzene rings are fused sharing two carbon atoms.
What is the term used to describe a compound with two or more rings in a bicyclic system?
-The term used is 'bicyclo' to describe such a compound.
How does the script illustrate the exploration of nomenclature rules for polycyclic compounds?
-The script uses ChemDoodle, a chemical drawing tool, to dynamically show how the nomenclature changes with the addition of substituents to different positions on the polycyclic structures.
What is the purpose of using ChemDoodle in the script to explore nomenclature rules?
-ChemDoodle is used to visually demonstrate how nomenclature rules apply to polycyclic structures and how the names change with the addition or movement of substituents.
How does the script define the numbering scheme for anthracene?
-The script defines the numbering scheme for anthracene by starting with the lowest possible number for the substituent and proceeding in a clockwise fashion around the outermost rings, then moving to the central rings.
What is the term used to describe a compound with three rings sharing a common vertex?
-The term used is 'bicyclo' followed by the number of carbons in each ring, starting with the largest, to describe such a compound.
How does the script handle the complexity of naming polycyclic aromatic hydrocarbons with multiple substituents?
-The script demonstrates the process of determining the parent chain and numbering the positions of substituents to assign the correct name according to IUPAC rules.
Outlines
🔍 Introduction to Polycyclic Structure Nomenclature
The script begins with an introduction to the nomenclature of polycyclic structures, emphasizing the importance of using tools like ChemDoodle to explore nomenclature rules interactively. It starts with a review of simple alkane nomenclature, explaining the process of finding the longest carbon chain and numbering to give substituents the lowest possible numbers. The script then transitions into the main topic of polycyclic structures, using naphthalene as an example to demonstrate how to name fused benzene rings and their derivatives with substituents in various positions.
📚 Nomenclature of Substituted Polycyclic Aromatic Compounds
This section delves into the nomenclature of polycyclic aromatic compounds, specifically focusing on naphthalene and its substituted forms. The script discusses how the position of substituents affects the naming, with examples of methyl groups attached at different positions on the naphthalene structure. It also introduces the concept of numbering to give the lowest set of locants to the substituents, and how the orientation of the molecule can influence the numbering scheme.
🌐 Exploring Biphenyl and Anthracene Nomenclature
The script moves on to discuss the nomenclature of biphenyl and anthracene compounds. It explains the difference between fused and connected rings, using ortho-phenyl toluene as an example to illustrate how substituents change the naming scheme. The script then covers anthracene, a three-ring structure, and demonstrates how to determine the numbering scheme by considering the molecule's symmetry and the position of substituents.
🔄 Understanding Spiro and Bicyclo Compound Nomenclature
The final section of the script introduces the nomenclature for Spiro and bicyclo compounds. It explains the use of the term 'spiro' for compounds with two rings connected by a single carbon, and how to name them by counting the carbons in each ring. The script also covers bicyclo compounds, where rings are connected by more than one carbon, and demonstrates the process of naming these structures by giving the numbers of carbons in each ring and the total number of carbons in the compound.
Mindmap
Keywords
💡Nomenclature
💡Polycyclic Structures
💡ChemDoodle
💡Benzene Rings
💡Fused Rings
💡Substituents
💡IUPAC Rules
💡Spiro Compounds
💡Bicyclo Compounds
💡Bridgehead Carbons
💡Aromatic Compounds
💡Aliphatic Compounds
Highlights
Introduction to using ChemDoodle for exploring nomenclature rules of polycyclic structures.
Review of basic alkane nomenclature to establish a common understanding.
Demonstration of how to identify the longest carbon chain in alkanes for naming.
Explanation of numbering alkanes to give substituents the earliest position.
Interactive feature of ChemDoodle allowing real-time structural modification and nomenclature exploration.
Naphthalene nomenclature and the significance of fused benzene rings.
Impact of substituent position on the naming of naphthalene derivatives.
Understanding identical positions in naphthalene due to molecular rotation.
Biphenyl nomenclature and the distinction from naphthalene based on ring connection.
Dynamics of changing parent names in biphenyls based on substituent addition.
Explanation of ortho, meta, and para relationships in substituted benzene rings.
Anthracene nomenclature and the process of numbering in polycyclic aromatic hydrocarbons.
Spiro compound nomenclature focusing on the number of carbons in each ring.
Bicyclo compound nomenclature and the method of numbering from the largest to smallest ring.
Practical application of nomenclature rules with the example of bicyclo[3.2.1]octane.
Visualization techniques for complex polycyclic structures to aid understanding.
Bicyclo[n.3.2]alkane nomenclature with an example of an undecane.
Final remarks on the utility of exploring nomenclature rules for polycyclic compounds.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: