Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Series
TLDRThis educational video script delves into Bohr's model of the atom, explaining electron movement in quantized energy levels around the nucleus. It covers the calculation of energy released or absorbed during electron transitions, using the Rydberg formula. The script also explores the relationship between photon energy, frequency, and wavelength, demonstrating how to determine the energy and frequency of a photon emitted or absorbed during these transitions. Finally, it discusses the Lyman, Balmer, and other series associated with different electron transitions and their corresponding light spectra, including visible light.
Takeaways
- 🌐 Bohr's model of the atom proposes that electrons move in circular orbits around the nucleus and can only occupy discrete energy levels.
- 🔢 Energy levels are quantized, meaning electrons can only have certain integer-based values and cannot exist between these levels.
- 📉 When an electron transitions from a higher to a lower energy level, it emits energy in the form of a photon.
- 📈 Conversely, an electron can only jump to a higher energy level by absorbing a photon with the correct energy.
- ⚖️ The energy change during electron transitions can be calculated using the equation: E = -2.178 × 10^-18 J * (1/n_final^2 - 1/n_initial^2).
- 🌌 The sign of the energy (positive or negative) indicates whether energy is absorbed or released, with negative values indicating emission.
- 🎼 The frequency of the emitted or absorbed photon can be found by dividing the energy by Planck's constant.
- 🌈 The wavelength of the photon is the speed of light divided by its frequency, and can be converted to nanometers for practical measurements.
- 🔮 To determine the energy level an electron will jump to after absorbing a photon, the energy of the photon is equated to the energy difference between levels using the Bohr model equation.
- ⚡ The greatest energy release occurs when an electron transitions to the lowest energy level from a higher one, typically from levels further out.
- 🌌 Different electron transitions are associated with different parts of the electromagnetic spectrum, with the Balmer series (n_final = 2) corresponding to visible light.
Q & A
What is the main concept of Bohr's model of the atom?
-Bohr's model of the atom proposes that electrons move around the nucleus in specific circular orbits or energy levels, and they can only occupy these quantized energy levels, not any arbitrary energy in between.
Why are the energy levels of electrons in Bohr's model quantized?
-The energy levels are quantized because electrons can only have discrete values and can only occupy certain orbits, not any arbitrary position in between the defined energy levels.
What happens when an electron falls from a higher energy level to a lower one?
-When an electron falls from a higher energy level to a lower one, it emits energy in the form of a photon.
How can the energy released or absorbed when an electron transitions between energy levels be calculated?
-The energy released or absorbed can be calculated using the equation E = -2.178 × 10^-18 joules * (1/n_final^2 - 1/n_initial^2), where n_initial is the original energy level and n_final is the new energy level.
Why is the energy value calculated for an electron transition negative when it falls to a lower energy level?
-The energy value is negative because it represents the energy released by the electron as it loses energy and falls to a lower energy level.
How is the frequency of the emitted or absorbed photon related to its energy?
-The frequency of the photon is calculated by dividing the energy of the photon by Planck's constant, which means the energy is directly proportional to the frequency.
What is the relationship between the speed of light, frequency, and wavelength of a photon?
-The speed of light is equal to the product of the wavelength and frequency of a photon. Mathematically, it is expressed as c = λν, where c is the speed of light, λ is the wavelength, and ν is the frequency.
How can you determine the energy level an electron will jump to after absorbing a photon?
-You can determine the energy level an electron will jump to by using the energy of the absorbed photon and solving the Bohr energy equation for the unknown energy level (n_final), ensuring that the result is an integer value.
Which electron transition involves the greatest release of energy according to the script?
-The electron transition that involves the greatest release of energy is the one where the electron falls from a higher energy level to the lowest energy level, which in the context of the script is the transition from n=3 to n=1.
Which electron transition will emit a photon in the visible light spectrum according to the script?
-The electron transition that will emit a photon in the visible light spectrum is associated with the Balmer series, where the final energy level is n=2.
What are the different series of electron transitions in the hydrogen atom spectrum?
-The different series are the Lyman series (ending at n=1), the Balmer series (ending at n=2), the Paschen series (ending at n=3), the Brackett series (ending at n=4), and the Pfund series (ending at n=5), each associated with different regions of the electromagnetic spectrum.
Outlines
🔬 Bohr's Atomic Model and Energy Quantization
This paragraph introduces Bohr's model of the atom, which posits that electrons orbit the nucleus in quantized energy levels. The electrons can only occupy specific orbits and cannot exist between these levels, leading to the concept of quantization where energy levels are discrete and integer-based. The paragraph also explains the process of energy emission or absorption when electrons transition between these levels, with energy released as a photon when an electron falls to a lower level and absorbed when it jumps to a higher level. The main equation for calculating the energy change during these transitions is introduced, along with an example calculation for an electron moving from the fourth to the second energy level in a hydrogen atom.
📡 Calculating Photon Frequency and Wavelength
The second paragraph delves into the calculation of the frequency and wavelength of a photon resulting from an electron transition. It explains how to use the energy of the emitted photon, Planck's constant, and the speed of light to determine the photon's frequency and wavelength. The process involves converting the energy from joules to a frequency in hertz and then using the speed of light to find the wavelength in nanometers. An example calculation is provided, demonstrating these steps for a photon resulting from an electron transition in a hydrogen atom.
🚀 Electron Transition and Energy Level Determination
This paragraph focuses on determining the energy level to which an electron will jump after absorbing a photon with a given wavelength. It outlines the process of calculating the energy of the photon using the wavelength and then using Bohr's energy equation to solve for the final energy level. The importance of correctly identifying whether the energy change is positive (absorption) or negative (emission) is emphasized. The paragraph guides through the mathematical steps to find the final energy level, using an example where an electron in the third energy level absorbs a photon with a specific wavelength.
⚡ Understanding Energy Release in Electron Transitions
The fourth paragraph discusses the concept of energy release during electron transitions, particularly highlighting which transitions result in the greatest energy release. It explains the process of identifying transitions from higher to lower energy levels and uses the energy equation to compare different transitions, illustrating how to calculate the energy change for each. The paragraph also provides a multiple-choice question to identify the transition with the greatest energy release, guiding the viewer through the elimination process based on the principles discussed.
🌈 Series of Electron Transitions and Associated Spectra
The final paragraph provides an overview of the different series of electron transitions in a hydrogen atom and their corresponding spectra. It explains the Lyman, Balmer, Paschen, Brackett, and Pfund series, associating each with a specific energy level transition and the resulting photon emission spectrum, ranging from ultraviolet to infrared. The paragraph also addresses a question regarding which transition would emit a photon in the visible light spectrum, identifying the Balmer series as the correct answer.
Mindmap
Keywords
💡Bohr's Model
💡Electron
💡Nucleus
💡Energy Levels
💡Quantized
💡Photon
💡Energy Equation
💡Frequency
💡Wavelength
💡Visible Light Spectrum
💡Lyman Series
💡Balmer Series
💡Paschen Series
Highlights
Bohr's model of the atom proposes that electrons move in circular orbits around the nucleus.
Electrons can only occupy discrete energy levels and not anywhere in between, indicating quantization of energy levels.
The energy levels are integer-based, with electrons unable to occupy fractional levels like 1.4 or 1.86.
When an electron falls from a high to a low energy level, it emits energy in the form of a photon.
An electron can only jump to a higher energy level by absorbing a photon with the right energy.
The energy released or absorbed when an electron moves between energy levels can be calculated using a specific equation.
The energy equation involves the Rydberg constant and the initial and final energy levels of the electron.
A negative answer for energy indicates that energy is released as the electron falls to a lower energy level.
The frequency of the emitted or absorbed photon can be calculated using Planck's constant and the energy of the photon.
The wavelength of a photon can be determined from its frequency using the speed of light.
Conversion from meters to nanometers is necessary to express the wavelength in nanometers.
An electron in the third energy level absorbs a photon with a specific wavelength, causing it to jump to a higher energy level.
The energy of a photon absorbed by an electron can be calculated from its wavelength.
The final energy level to which an electron jumps after absorbing a photon can be determined by solving an equation.
The electron transition involving the greatest release of energy is from a higher energy level to the lowest, which is n=1.
Different electron transitions are associated with different series: Lyman, Balmer, Paschen, Brackett, and Pfund, each with a specific spectral range.
The Balmer series is associated with the visible light spectrum, making it the answer for transitions emitting visible light.
Transcripts
Browse More Related Video

Bohr's Atomic Model
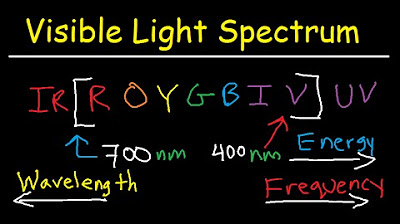
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry
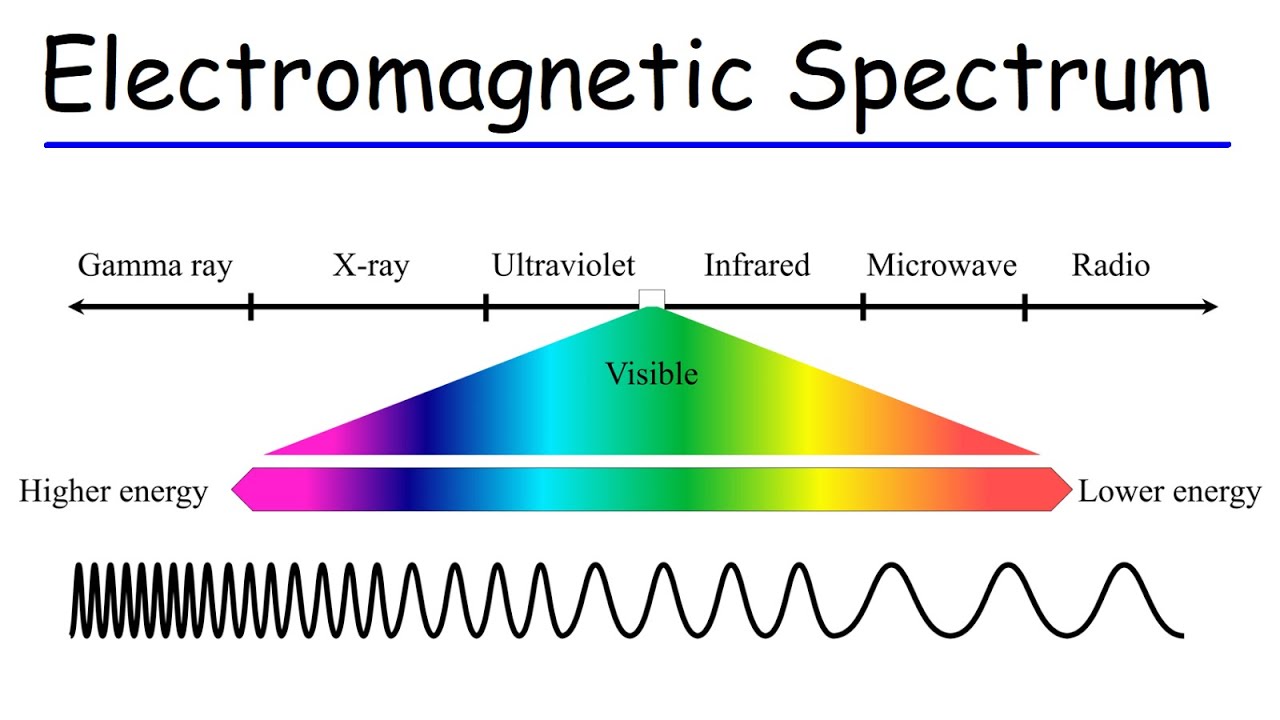
Electromagnetic Spectrum - Basic Introduction

Emission and Absorption Spectra

Lecture 9 - Some relations from the Bohr's theory
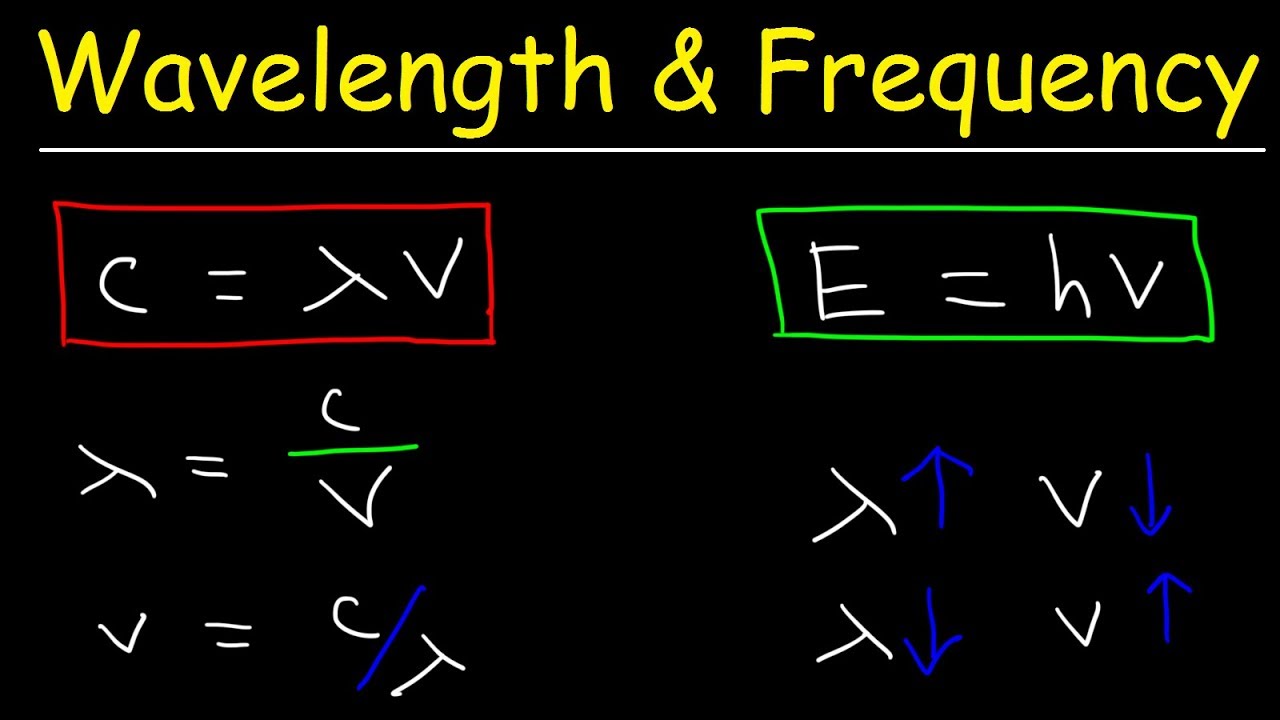
Speed of Light, Frequency, and Wavelength Calculations - Chemistry Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: