Lecture 7 Planck's Quantum Theory
TLDRThis script delves into Planck's quantum theory, addressing phenomena like the photoelectric effect and spectral lines that classical theories could not explain. It introduces the concept of a 'blackbody', an idealized object that absorbs all incident radiation and emits 'blackbody radiation'. Planck's constant is highlighted, linking energy to the frequency of light through the formula E = hν. The script also previews Bohr's atomic theory, touching on fundamental formulas for columbic force, centripetal force, and angular momentum, setting the stage for a deeper exploration in subsequent lectures.
Takeaways
- 🌟 Planck's quantum theory was introduced to explain phenomena that Maxwell's theory of continuous energy flow could not, such as the photoelectric effect and spectral lines from excited atomic gases.
- 📸 The photoelectric effect is the emission of electrons from a metal surface when light of a certain wavelength is incident upon it, which was inexplicable by Maxwell's theories.
- 🌈 Spectral lines, particularly the 'nine spectrum,' are emitted by excited atomic gases and are used to identify elements, but they could not be explained by Maxwell's theory.
- 🔥 Blackbody is an idealized object that absorbs all incident radiation and is a perfect emitter of blackbody radiation, which is a key concept in understanding Planck's theory.
- ⚫ Planck introduced the constant 'h' (Planck's constant) to quantify the relationship between energy and frequency, with the formula E = hν, where ν is the frequency of the wave.
- 🔢 Planck's constant (h) is approximately 6.626 x 10^-34 joule-seconds, a fundamental value in quantum physics.
- 🔄 The energy of light can also be expressed in terms of its wavelength (λ) using the formula E = hc/λ, where c is the speed of light and h is Planck's constant.
- 🌌 The wave number (1/λ) is an alternative way to express the relationship between energy and wavelength in quantum physics.
- 🔋 Bohr's atomic theory, which builds on Planck's quantum theory, introduces formulas for understanding the behavior of electrons in atoms, such as the Coulombic force (electrostatic force).
- 🌀 The centripetal force experienced by a particle in circular motion is given by mv^2/r, where m is the mass, v is the velocity, and r is the radius of the orbit.
- 🎯 Angular momentum is defined as the product of the radius of the orbit (R) and the product of mass (M) and velocity (V), expressed as L = mvR.
Q & A
What was the main limitation of Maxwell's theory of electromagnetism?
-Maxwell's theory could explain the phenomena of light very well, but it could not account for certain phenomena such as the photoelectric effect and the emission of spectral lines by excited atomic gases.
What is the photoelectric effect?
-The photoelectric effect is the emission of electrons from a metal surface when it is exposed to light of a certain wavelength. The emitted electrons possess kinetic energy.
Why was the photoelectric effect a challenge for Maxwell's theory?
-The photoelectric effect could not be explained by Maxwell's theory because it assumed a continuous flow of energy, whereas the photoelectric effect involves the emission of electrons in discrete packets, which contradicts the continuous flow concept.
What are spectral lines and why were they problematic for Maxwell's theory?
-Spectral lines are the specific wavelengths of light emitted by excited atomic gases, which help in the identification of elements. They were problematic for Maxwell's theory because it could not explain the discrete nature of these lines.
What is a blackbody and why is it considered ideal?
-A blackbody is an idealized object that absorbs all incident radiation without reflecting any. It is considered ideal because it absorbs 100% of the radiation falling on it, which does not occur in real life.
What is the significance of blackbody radiation in the context of Planck's quantum theory?
-Blackbody radiation is the radiation emitted by a blackbody, which follows a specific spectrum. Planck's quantum theory was developed to explain the observed blackbody radiation spectrum, which could not be explained by classical physics.
What is Planck's constant and what is its value?
-Planck's constant, denoted by H, is a fundamental physical constant that relates the energy of a photon to its frequency. Its value is approximately 6.626 x 10^-34 joule-seconds.
How does Planck's quantum theory relate energy to the frequency of light?
-According to Planck's quantum theory, the energy of a photon is directly proportional to its frequency. This relationship is expressed by the formula E = Hν, where E is the energy, H is Planck's constant, and ν is the frequency of the light.
What is the formula for energy in terms of the speed of light, Planck's constant, and wavelength?
-The formula for energy in terms of the speed of light (c), Planck's constant (H), and wavelength (λ) is E = hc/λ, where c is the speed of light, approximately 3 x 10^8 meters per second.
What is Bohr's atomic theory and how does it relate to Planck's quantum theory?
-Bohr's atomic theory is a model of the atom where electrons orbit the nucleus in discrete energy levels. It builds upon Planck's quantum theory by applying the concept of quantized energy levels to the behavior of electrons in atoms.
What are the three main formulas mentioned in the script that are essential for understanding Bohr's theory?
-The three main formulas mentioned are: 1) Coulomb's law for electrostatic force (F = kQ1Q2/R^2), 2) Centrifugal force formula (F = mv^2/R), and 3) Angular momentum formula (L = mvR).
Outlines
🌟 Planck's Quantum Theory and Photoelectric Effect
This paragraph introduces Planck's quantum theory as a response to the limitations of Maxwell's theory of continuous energy flow, which failed to explain certain phenomena like the photoelectric effect and spectral lines of excited atomic gases. The photoelectric effect is described as the ejection of electrons from a metal surface when light of a certain wavelength is incident, resulting in electrons with kinetic energy. The paragraph also introduces the concept of a blackbody—an ideal body that absorbs all incident radiation—and the blackbody radiation that Planck's formulas help to understand, with energy being directly proportional to the frequency of light. Planck's constant (H = 6.626 × 10^-34 joule-seconds) is introduced as a fundamental constant in these formulas.
🔬 Bohr's Atomic Theory and Fundamental Forces
The second paragraph delves into Bohr's atomic theory, setting the stage for a more detailed discussion in a subsequent lecture. It presents three key formulas essential for understanding the theory: Coulomb's law for electrostatic force between two charges, the formula for centripetal force experienced by a particle in circular motion, and the definition of angular momentum. Coulomb's force is given by the formula K * (Q1 * Q2) / R^2, where Q1 and Q2 are charges and R is the distance between them. Centrifugal force is described with the formula M * V^2 / R, relating the mass (M), velocity (V), and radius (R) of the orbit. Angular momentum is defined as the product of the radius and the product of mass and velocity (R * M * V). These concepts are foundational for grasping Bohr's model of the atom.
Mindmap
Keywords
💡Planck's Quantum Theory
💡Photoelectric Effect
💡Spectral Lines
💡Blackbody
💡Blackbody Radiation
💡Planck's Constant (H)
💡Wave Number
💡Bohr's Atomic Theory
💡Coulombic Force
💡Centrifugal Force
💡Angular Momentum
Highlights
Planck's quantum theory was introduced to address phenomena that Maxwell's continuous energy flow theory could not explain.
The photoelectric effect is a phenomenon where light incident on a metal surface ejects electrons with kinetic energy.
Spectral lines emitted by excited atomic gases, known as the line spectrum, could not be explained by Maxwell's theories.
A blackbody is an ideal body that absorbs all incident radiation and does not exist in real life.
Blackbody radiation is the term for the radiation emitted by a blackbody, which is an ideal emitter.
Planck introduced the concept that energy is directly proportional to the frequency of light, introducing Planck's constant (H).
Planck's constant (H) is approximately 6.626 x 10^-34 joule-seconds.
The energy of a wave can be expressed as E = hν, where h is Planck's constant and ν is the frequency.
The relationship between the speed of light, frequency, and wavelength is given by c = λν, where c is the speed of light.
The formula E = hc/λ relates the energy of light to its frequency and wavelength.
The wave number, represented as 1/λ, is a term used in the formula for energy of light.
Bohr's atomic theory will be studied in detail in the next lecture, introducing key formulas for understanding the theory.
Coulomb's law describes the electrostatic force between two charges and is given by the formula F = kQ1Q2/R^2.
Centripetal force, experienced by a particle in circular motion, is given by F = mv^2/R.
Angular momentum is defined as the product of the radius and the product of mass and velocity (L = R * mv).
Transcripts
Browse More Related Video

Einstein's Quantum Physics Theory That Proved Him Wrong | The Secrets Of Quantum Physics | Spark

Bohr's Atomic Model

Lecture 1 | New Revolutions in Particle Physics: Basic Concepts
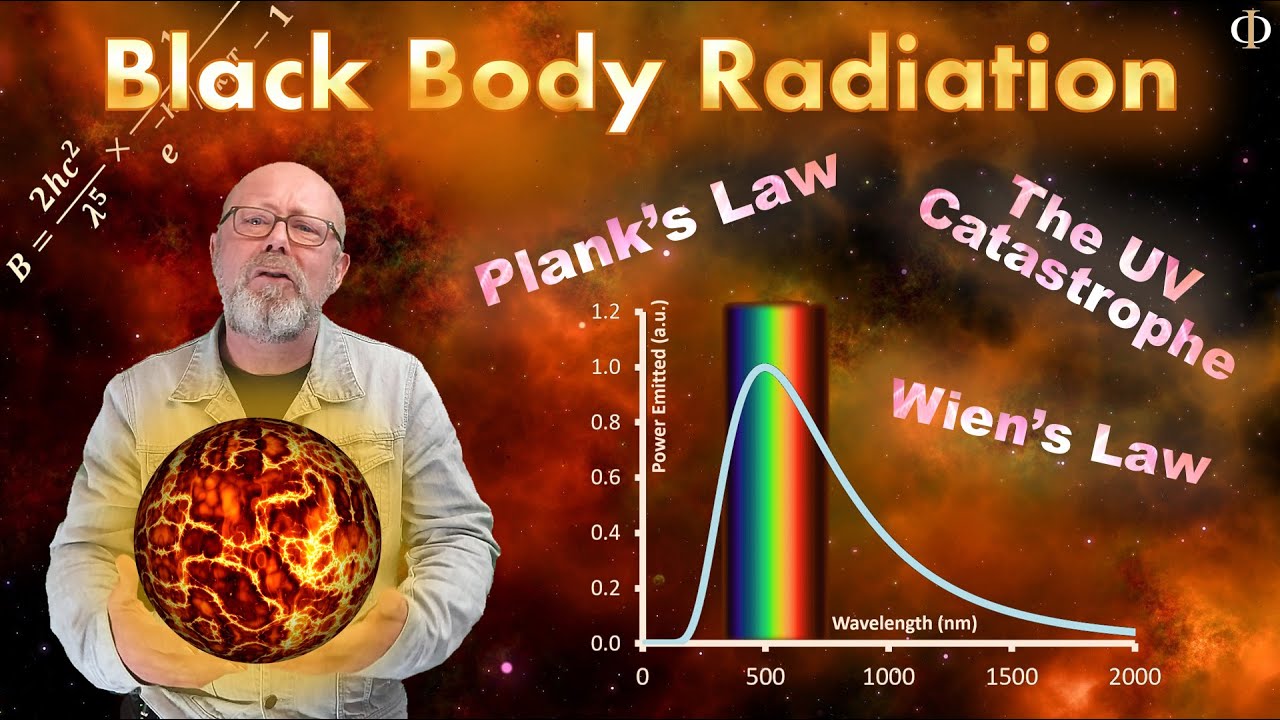
Black Body Radiation - Understanding the black body spectra using classical and quantum physics

Photons

The Birth of Quantum Mechanics
5.0 / 5 (0 votes)
Thanks for rating: