CO2 Phase Changes | Danfoss Climate Solutions
TLDRThis Dan Foss video explores the fascinating phases of CO2, focusing on its unique refrigerant properties and phase changes influenced by pressure and temperature. From the triple point, where solid, liquid, and vapor CO2 coexist, to the supercritical phase, the video delves into CO2's historical use and its current resurgence as a safe, efficient refrigerant and solvent, especially in the food industry, highlighting its high-pressure operation and narrow temperature range.
Takeaways
- 🌡️ The video discusses the different phases of CO2 and how its phase changes can be induced by altering pressure or temperature.
- 🧩 CO2, also known as R744, operates at higher pressures and a narrower temperature range compared to other refrigerants like R134a and R717.
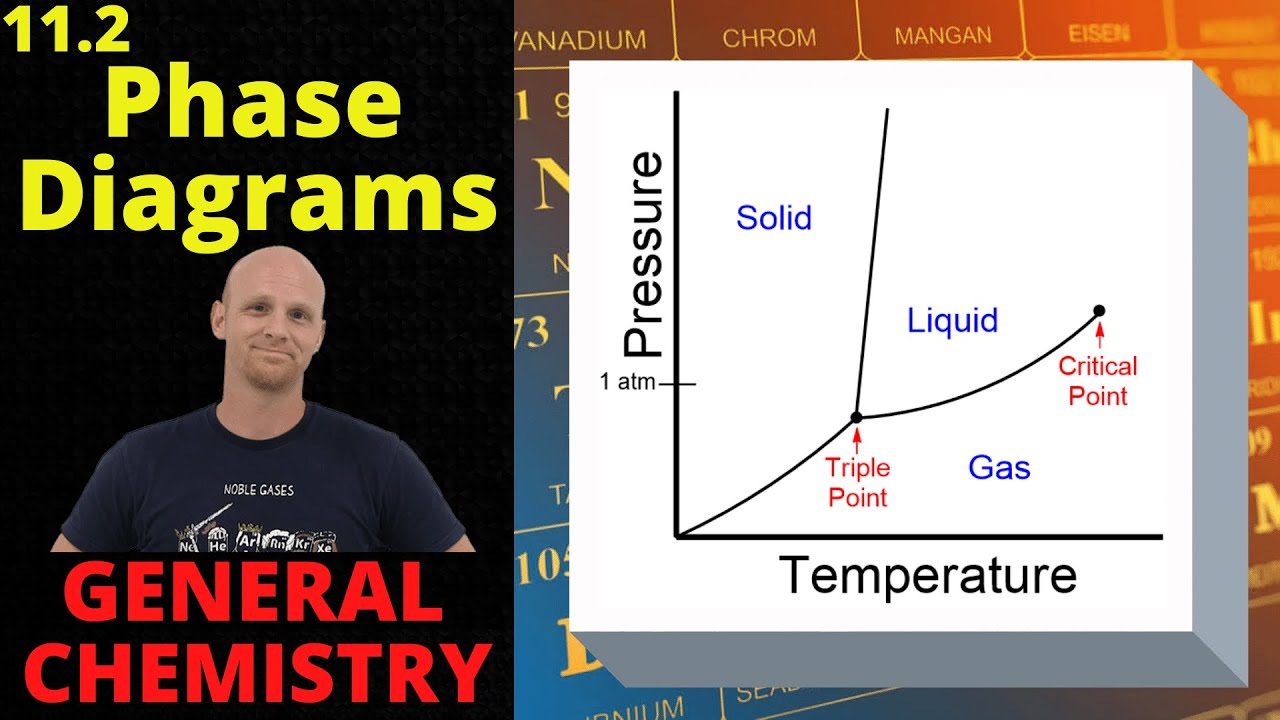
- 📈 The pressure at CO2's triple point is high, and the temperature at its critical point is low, which are important considerations for its use as a refrigerant.
- 🕰️ CO2 was first used as a refrigerant in 1850, experienced a peak in the 1920s and 1930s, and made a comeback in 1993 after Danfoss observed its phase changes.
- 🔬 The temperature-pressure diagram, or phase diagram, for pure CO2 is essential for understanding the conditions under which CO2 exists in different phases.
- 💧 At the triple point, CO2 can exist in solid, liquid, and vapor phases simultaneously, which occurs at a specific pressure and temperature.
- ❄️ Solid CO2, or dry ice, has a surface temperature of -109.12°F at standard atmospheric pressure.
- 🔥 The critical point of CO2 is where the distinction between liquid and vapor phases disappears, forming a supercritical phase with unique properties.
- 🌡️ The supercritical phase of CO2 has a density similar to liquids but with a much lower viscosity and higher diffusivity, making it useful as a solvent.
- 🍽️ Supercritical CO2 is of interest for use as a safe and effective solvent, particularly in the food industry for processes like extraction.
- 📊 The video demonstrates the phase changes of CO2 through an observation cell designed to withstand high pressures, illustrating the transition from liquid to supercritical phases.
Q & A
What is the main focus of the video produced by Dan Foss?
-The video focuses on discussing the different phases of CO2 and how two of its phase changes can be induced by altering pressure or temperature.
What are some unique characteristics of CO2 as a refrigerant compared to R134a and R717?
-CO2 operates at a much higher pressure and across a narrower temperature range than R134a and R717, and it has a high triple point pressure and a low critical point temperature.
When was the application of CO2 as a refrigerant first introduced?
-The application of CO2 as a refrigerant was first introduced in 1850.
Why did CO2 refrigeration technology disappear from the market between 1950 and 1960?
-The script does not provide a specific reason for the disappearance of CO2 refrigeration technology from the market during that period.
What was the significance of the observation cell constructed by Danfoss in 1993?
-The observation cell was designed to observe CO2 phase changes and marked a major comeback for CO2 refrigerant technology after its decline.
What is the maximum pressure the observation cell is designed to withstand?
-The observation cell is designed to withstand pressures up to 2030.53 psia.
What does the colored area in the temperature-pressure diagram represent for pure CO2?
-The colored areas represent the temperature and pressure limits at which the different phases of CO2—vapor, liquid, solid, and supercritical—exist.
What is the triple point of CO2, and what does it signify?
-The triple point is the only pressure and temperature combination at which solid, liquid, and vapor CO2 can exist simultaneously in equilibrium.
What is the critical point of CO2, and what happens at this point?
-The critical point is the pressure and temperature combination at which the distinction between liquid and vapor phases disappears, and only a single supercritical phase exists.
How is supercritical CO2 used in practical applications?
-Supercritical CO2 has properties that make it a safe and effective solvent, particularly for food products, due to its ability to act as a solvent while having a lower viscosity and higher diffusivity than most liquids.
What are some properties of supercritical CO2 that make it interesting for various applications?
-Supercritical CO2 has a density similar to many liquids but nearly 2.5 times higher than its vapor density at 68 degrees Fahrenheit, a lower viscosity than most liquids, and a higher diffusivity, making it suitable for extraction processes.
Outlines
🌡️ CO2 Phase Changes and Refrigerant Characteristics
This section of the video script delves into the various phases of carbon dioxide (CO2), focusing on how altering pressure or temperature can induce phase changes. It introduces CO2 as a refrigerant, highlighting its unique properties such as high operating pressure and a narrower temperature range compared to R134a and r717. The script also touches on the history of CO2 as a refrigerant, mentioning its initial introduction in 1850, the peak in the 1920s and early 1930s, and its resurgence in 1993. The importance of understanding the triple point and critical point of CO2 for its use as a refrigerant is emphasized. The video uses a specially designed observation cell by Dan Foss to visually demonstrate these phase changes, including the transition through the triple point where CO2 turns into solid, liquid, and vapor forms simultaneously.
🔥 Exploring the Supercritical Phase of CO2
The second part of the script discusses the supercritical phase of CO2, explaining the critical point as the unique pressure and temperature at which the distinction between liquid and vapor phases vanishes. The video demonstrates the process of heating CO2 to surpass its critical point, resulting in a single supercritical phase with properties of both liquid and gas. The supercritical CO2 is highlighted for its potential as a solvent, particularly in the food industry, due to its low viscosity and high diffusivity. The script concludes by showing the process of reducing pressure and temperature to revert from the supercritical phase back to the liquid and vapor equilibrium, inviting viewers to contact Dan Foss for more information.
Mindmap
Keywords
💡CO2
💡Phase Changes
💡Triple Point
💡Critical Point
💡Refrigerant
💡Pressure
💡Temperature
💡Supercritical Fluid
💡Danfoss
💡Venting
💡Phase Diagram
Highlights
The video discusses different phases of CO2 and how phase changes occur by altering pressure or temperature.
CO2, also known as R744, operates at higher pressures and narrower temperature ranges compared to R134a and r717.
The high triple point pressure and low critical point temperature of CO2 are crucial for its use as a refrigerant.
CO2 as a refrigerant was introduced in 1850 and experienced a comeback in 1993 after Danfoss' observation cell for phase changes.
The observation cell is designed to withstand pressures up to 2030.53 psia for studying CO2 phase changes.
The temperature-pressure diagram for pure CO2 shows the limits of CO2's different phases.
The triple point is the only condition where solid, liquid, and vapor CO2 can coexist in equilibrium.
The process of CO2 cooling involves forced evaporation and passing through the triple point.
Solid CO2, or dry ice, has a surface temperature of minus 109.12 degrees Fahrenheit at standard atmospheric pressure.
The supercritical phase of CO2 is reached by increasing temperature and pressure beyond the critical point.
At the critical point, the distinction between liquid and vapor CO2 phases disappears, forming a single phase.
Supercritical CO2 has properties of both a liquid and a gas, making it useful as a solvent.
Supercritical CO2 is of interest for use in food products due to its safety and effectiveness.
The video demonstrates the process of reducing supercritical CO2 pressure and temperature to return to liquid and vapor equilibrium.
Danfoss encourages viewers to reach out to their local sales organization for more information on CO2 refrigeration technology.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: