CO2 Phase Changes | Danfoss Cool | Video english
TLDRThis video by Dan Foss explores the unique properties of carbon dioxide (CO2) as a refrigerant, highlighting its different phases and phase changes influenced by pressure and temperature. It discusses CO2's high-pressure operation and narrow temperature range compared to common refrigerants like R134a and R717. The video uses a specially designed observation cell to demonstrate the triple and critical points of CO2, showing how it transitions between solid, liquid, vapor, and supercritical states. The supercritical phase of CO2, with its solvent properties and low viscosity, is of particular interest for applications in the food industry.
Takeaways
- 🌟 CO2, also known as carbon dioxide, can exist in various phases and undergoes phase changes with alterations in pressure or temperature.
- 🔧 CO2 operates at higher pressures and a narrower temperature range compared to common refrigerants like R134a and R717.
- 📚 The use of CO2 as a refrigerant dates back to 1850, with a peak in the 1920s and 1930s, but almost disappeared between 1950 and 1960 before being revived in 1993.
- 🔬 A specially designed observation cell by Dan Foss is used to study CO2 phase changes, capable of withstanding pressures up to 140 bar gauge.
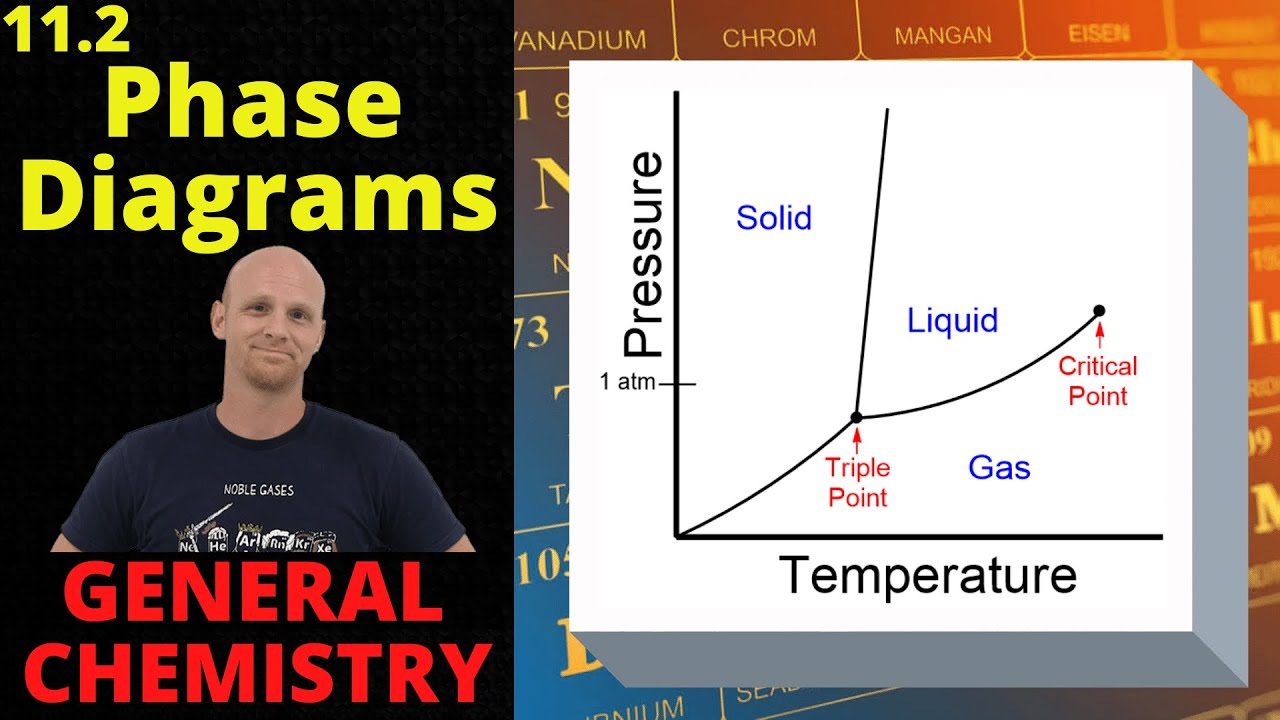
- 📈 The phase diagram (temperature-pressure diagram) is essential for understanding the conditions under which CO2 exists in vapor, liquid, solid, and supercritical phases.
- 🧊 The triple point of CO2 is a unique condition where solid, liquid, and vapor CO2 coexist in equilibrium at a specific pressure and temperature.
- ❄️ Solid CO2, or dry ice, has a surface temperature of -78.4°C at standard atmospheric pressure.
- 🌡️ The critical point of CO2 is the combination of pressure and temperature at which the densities of liquid and vapor CO2 become equal, forming a supercritical phase.
- 🌡️ Supercritical CO2 has unique properties, such as acting as a solvent with lower viscosity and higher diffusivity than most liquids, making it useful for various applications including food products.
- 🛠️ The phase changes of CO2, including the triple point and supercritical phase, are demonstrated through controlled heating and cooling within the observation cell.
- 📝 For further information on CO2 refrigeration technology, one should contact their local Danfoss sales organization.
Q & A
What is the main subject of the video?
-The main subject of the video is the different phases of carbon dioxide (CO2) and how phase changes occur by altering pressure or temperature.
What are the common names for the substance discussed in the video?
-The common names for the substance discussed in the video are carbon dioxide, CO2, and R744.
How does CO2 as a refrigerant compare to R134a and R717 in terms of pressure and temperature range?
-CO2 operates at a much higher pressure but across a narrower temperature range compared to both R134a and R717.
What are the unique characteristics of CO2's triple and critical points?
-The pressure at CO2's triple point is high, and the temperature at the critical point is low, which are important considerations when using CO2 as a refrigerant.
When was the application of CO2 as a refrigerant first introduced?
-The application of CO2 as a refrigerant was first introduced in 1850.
What happened to CO2 refrigeration technology between 1950 and 1960?
-Between 1950 and 1960, CO2 refrigeration technology virtually disappeared from the market.
In what year was CO2 refrigeration technology revived, and why was it observed?
-CO2 refrigeration technology was revived in 1993 to observe the phase changes of CO2.
What is the purpose of the observation cell used in the video?
-The observation cell, designed and constructed by Dan Foss, is used to withstand pressures up to 140 bar gauge and to observe the different phases of CO2.
What is the triple point in the context of the phase diagram for CO2?
-The triple point is the only pressure and temperature combination at which solid, liquid, and vapor CO2 can exist simultaneously in equilibrium.
What is the significance of the critical point in the phase diagram for CO2?
-The critical point is the pressure and temperature combination at which the density of both the liquid and vapor are equal, and the distinction between the two phases disappears, leading to a single supercritical phase.
What are some of the user-friendly properties of supercritical CO2?
-Supercritical CO2 has properties of a liquid, allowing it to act as a solvent, but its viscosity is much lower than most liquids, and its diffusivity is higher, making it of interest for use in various applications, including as a safe and effective solvent for food products.
How can the phase changes of CO2 be observed in the specially designed cell?
-The phase changes of CO2 can be observed by altering the temperature and pressure within the cell, allowing it to pass through the triple point into solid CO2 and through the critical point into the supercritical phase.
Outlines
🌡️ CO2 Phase Changes and Refrigeration Characteristics
This paragraph introduces the various phases of carbon dioxide (CO2), focusing on its unique characteristics as a refrigerant. CO2 operates at higher pressures and narrower temperature ranges compared to other refrigerants like R134a and R717. The video script explains the importance of understanding the triple point and critical point when using CO2 in refrigeration systems. It also provides historical context, noting the peak of CO2 refrigeration technology in the 1920s and 1930s, and its revival in 1993. The use of an observation cell to study phase changes is highlighted, along with the temperature-pressure diagram that defines the limits of CO2's different phases.
❄️ The Triple and Supercritical Points of CO2
This section delves into the specific conditions that define the triple and supercritical points of CO2. The triple point is the unique pressure and temperature at which solid, liquid, and vapor CO2 can coexist in equilibrium. The paragraph describes the process of reaching this point by reducing pressure and temperature, resulting in the formation of solid CO2, also known as dry ice. The supercritical phase is then explored, where CO2 reaches a state beyond its critical point, characterized by equal densities of liquid and vapor, leading to a single phase with unique properties. The supercritical CO2 is noted for its potential as a solvent due to its combined properties of liquid and gas.
🔍 Applications and Interest in Supercritical CO2
The final paragraph discusses the practical applications and growing interest in supercritical CO2, particularly as a safe and effective solvent for food products. It describes the process of reducing pressure and temperature to transition from the supercritical phase back to liquid and vapor states. The paragraph concludes by inviting viewers to seek further information from their local Danfoss sales organization, emphasizing the company's role in providing insights into CO2 refrigeration technology.
Mindmap
Keywords
💡Carbon Dioxide (CO2)
💡Phase Changes
💡Refrigerants
💡Triple Point
💡Critical Point
💡Pressure
💡Temperature Range
💡Supercritical Fluid
💡Phase Diagram
💡Enthalpy
💡Danfoss
Highlights
CO2, also known as carbon dioxide, is discussed in various phases and how phase changes occur through pressure or temperature alterations.
CO2 operates at higher pressures and narrower temperature ranges compared to refrigerants like R134a and R717.
The high triple point pressure and low critical point temperature of CO2 are crucial for its use as a refrigerant.
The history of CO2 as a refrigerant dates back to 1850, with peak usage in the 1920s and 1930s, and a revival in 1993.
A specially designed observation cell by Dan Foss is used to study CO2 phase changes, capable of withstanding up to 140 bar gauge pressures.
The temperature-pressure diagram, or phase diagram, illustrates the existence of CO2 in vapor, liquid, solid, and supercritical phases.
The triple point of CO2 is the unique condition where solid, liquid, and vapor coexist in equilibrium.
The process of reaching the triple point involves cooling CO2 through forced evaporation and adjusting pressure and temperature.
Dry ice, or solid CO2, has a surface temperature of -78.4°C at standard atmospheric pressure.
The supercritical phase of CO2 is characterized by the disappearance of the distinction between liquid and vapor phases.
The critical point is where the densities of liquid and vapor CO2 become equal, forming a single phase.
Supercritical CO2 has properties of a liquid but with lower viscosity and higher diffusivity, making it a versatile solvent.
The use of supercritical CO2 as a solvent is of particular interest for food products due to its safety and effectiveness.
The video demonstrates the transition of CO2 through its triple point and into the supercritical phase by adjusting temperature and pressure.
The observation cell is filled with liquid CO2 under controlled conditions to study its behavior at various stages.
The video concludes with a demonstration of the re-establishment of liquid and vapor equilibrium after passing through the critical point.
For further information on CO2 refrigeration technology, viewers are directed to contact their local Danfoss sales organization.
Transcripts
Browse More Related Video

CO2 Phase Changes | Danfoss Climate Solutions

Going supercritical.

Phase diagrams | States of matter and intermolecular forces | Chemistry | Khan Academy

Phase Diagrams | Phase Diagram of Water and Phase Diagram of Carbon Dioxide
11.2 Phase Diagrams | General Chemistry

Thermodynamics - Explaining the Critical Point
5.0 / 5 (0 votes)
Thanks for rating: