Expressing Concentration by Mass Percent
TLDRIn this educational video, Professor Dave explains the concept of mass percent, a fundamental measure of concentration in chemistry. He clarifies how mass percent is calculated as the ratio of a solute's mass to the total mass of a solution, expressed as a percentage. The video provides practical examples, such as household cleaning products like bleach and bathroom tile cleaner, to illustrate how mass percent is used to describe the concentration of substances in everyday life. Additionally, it demonstrates the utility of mass percent in laboratory settings, where it simplifies the preparation of solutions without complex stoichiometric calculations.
Takeaways
- 🧪 Mass percent is a way to describe the concentration of a solute in a solution relative to the total mass of the solution.
- 💧 Molarity is another method to express concentration, which is measured in moles of solute per liter of solution.
- 📦 Mass percent is commonly used for everyday substances and in situations where measuring by mass is more practical than by moles.
- 🧴 Household cleaning products often list their active ingredient concentration in mass percent, making it easier for the general public to understand.
- 🧼 An example given is bleach, where the concentration of sodium hypochlorite is listed as 7.4% by mass.
- 🔢 To calculate mass percent, divide the mass of the component by the total mass of the solution and multiply by 100.
- 🧴 Another example is a bathroom tile cleaner containing 165g of hydrochloric acid and 790g of water, which results in a 17.2% mass percent of HCl.
- 🧪 Mass percent is also useful in a laboratory setting for quickly preparing solutions without complex stoichiometric calculations.
- 🥣 If you want to make a 10% sodium hydroxide solution by mass, you would weigh out 10g of sodium hydroxide and add 90g of water, resulting in a 100g solution.
- 🔄 The concept of mass percent has a wide range of applications, from everyday use to industrial and scientific applications.
Q & A
What is mass percent in chemistry?
-Mass percent is the ratio of the mass of a component to the total mass of the solution, expressed as a percentage.
How is a solution formed?
-A solution is formed by placing some solute into some solvent.
What terms are used to describe the concentration of a solution?
-If there is very little solute, the solution is described as dilute. If there is a lot of solute, the solution is described as concentrated.
How is molarity used to report concentrations?
-Molarity is expressed in moles of solute per liter of solution.
Why might mass percent be used instead of molarity in some situations?
-Mass percent might be used because it can be an easier way to measure and report concentration, especially for certain situations in the laboratory or for everyday substances.
How is mass percent typically listed?
-Mass percent is often listed by naming the substance followed by a percent symbol, with the assumption that it is describing mass.
Give an example of how mass percent might be used in household products.
-For example, the concentration of sodium hypochlorite in a typical bottle of bleach might be listed as 7.4%.
How do you calculate mass percent using hydrochloric acid in bathroom tile cleaner?
-To calculate mass percent, use the mass of hydrochloric acid (165 grams) and the total mass of the solution (165 grams HCl + 790 grams water = 955 grams). The mass percent is (165/955) * 100 = 17.2%.
Why is the concept of mass percent useful in the lab?
-Mass percent is useful because it simplifies the preparation of solutions without needing to perform stoichiometric calculations.
How would you make a ten percent sodium hydroxide solution by mass?
-Measure out ten grams of sodium hydroxide pellets and add 90 milliliters (90 grams) of water to make a 100 gram solution that is ten percent sodium hydroxide by mass.
Outlines
🔬 Understanding Mass Percent in Chemistry
This paragraph introduces the concept of mass percent in chemistry. It explains that a solution's concentration depends on the amount of solute relative to the solvent. Solutions with little solute are termed dilute, while those with a lot of solute are concentrated. Molarity, which measures concentration in moles of solute per liter of solution, is mentioned. However, mass percent is introduced as a more practical way to describe concentrations for certain laboratory situations and everyday substances, expressed as the ratio of the solute's mass to the total solution's mass.
🧪 Practical Applications of Mass Percent
The paragraph provides practical applications of mass percent, particularly in household cleaning products. For example, a typical bleach bottle might list the concentration of sodium hypochlorite as 7.4%, meaning 7.4 grams of sodium hypochlorite per 100 grams of solution. This method of describing concentration is more accessible to the general public who may not be familiar with moles or molarity.
🏠 Calculating Mass Percent in Household Products
This paragraph demonstrates how to calculate mass percent using a household example. It describes a bathroom tile cleaner containing 165 grams of hydrochloric acid and 790 grams of water. By using the mass percent equation, the concentration of hydrochloric acid is calculated to be 17.2%. This example illustrates how to determine the mass percent of a component in a solution and compare it to other products.
🔬 Lab Applications of Mass Percent
The paragraph highlights the usefulness of mass percent in laboratory settings. It describes how to create an aqueous solution of 10% sodium hydroxide by mass. By measuring 10 grams of sodium hydroxide and adding 90 milliliters of water (since water has a density of 1 gram per milliliter), a 100-gram solution with the desired concentration is made without complex calculations. This method simplifies preparation of solutions in lab settings.
🧪 Versatility and Importance of Mass Percent
The final paragraph emphasizes the broad applications of mass percent in both public and industrial contexts. It reiterates that mass percent is a practical way to describe concentrations, making it easier for both laypeople and scientists to understand and use. The concept is applicable in everyday products and laboratory settings, providing a reliable and straightforward method to measure and report concentrations.
Mindmap
Keywords
💡mass percent
💡solution
💡solute
💡solvent
💡concentration
💡molarity
💡laboratory
💡household cleaning products
💡sodium hypochlorite
💡hydrochloric acid
💡sodium hydroxide
Highlights
Introduction to mass percent in chemistry as a method to express solution concentration.
Explanation of dilute and concentrated solutions based on solute amount.
Comparison between molarity and mass percent for expressing concentration.
Definition of mass percent as a ratio of component mass to total solution mass.
Practicality of mass percent for non-scientists in everyday substances.
Example of household cleaning products using mass percent for concentration.
Illustration of mass percent with a bottle of bleach containing 7.4% sodium hypochlorite.
Calculation method for mass percent using the equation and an example.
Example calculation of hydrochloric acid mass percent in a bathroom tile cleaner.
Result of the calculation showing 17.2% mass percent of hydrochloric acid.
Utility of mass percent in the laboratory for simplifying preparation of solutions.
Demonstration of preparing a 10% sodium hydroxide solution by mass.
Explanation of how mass percent simplifies solution preparation without calculations.
Discussion on the wide applications of mass percent in both public and industry.
Encouragement to check comprehension on the concept of mass percent.
Transcripts
Browse More Related Video

Calculate %m/m (Percent by Mass of a solution)

How to calculate percent concentration | Percent mass | Percent volume | Percent mass-volume - Dr K
Mass Percent of a Solution Made Easy: How to Calculate Mass % or Make a Specific Concentration

Calculate %m/v, Mass-Volume Percent + 2 Examples
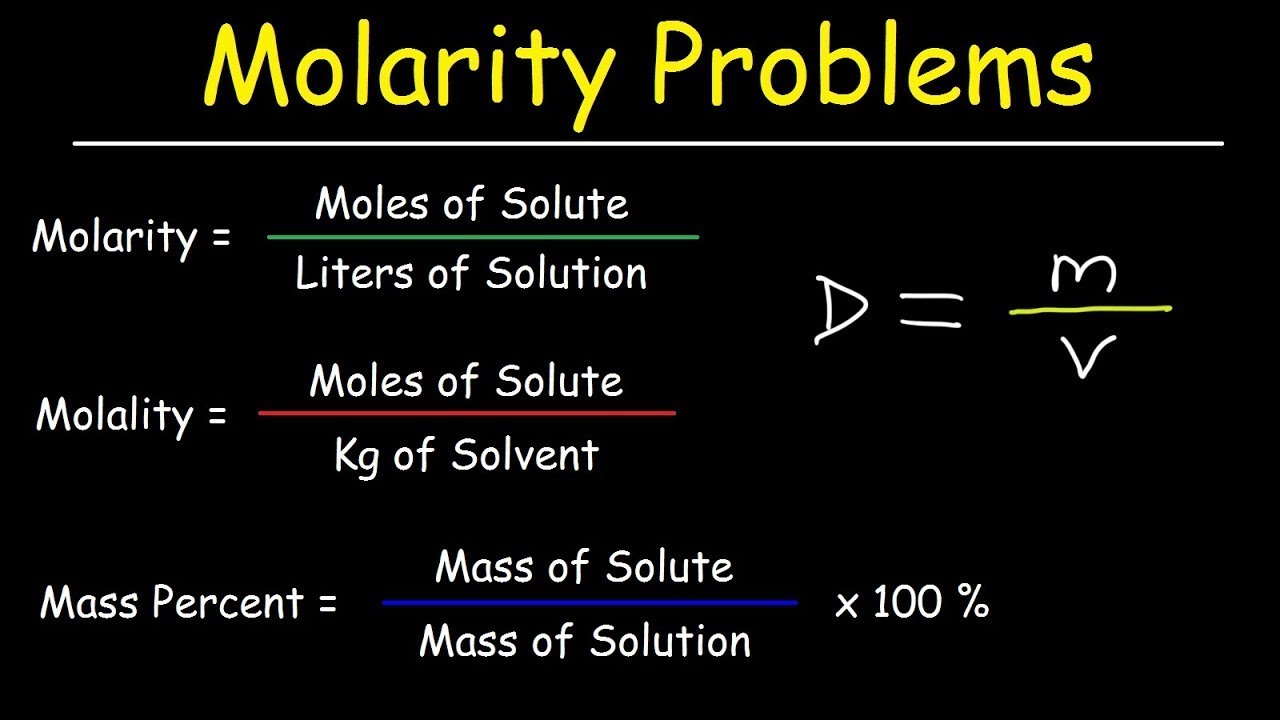
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration Problems

How to Calculate Mass Percent of a Solution
5.0 / 5 (0 votes)
Thanks for rating: