Mass Percent of a Solution Made Easy: How to Calculate Mass % or Make a Specific Concentration
TLDRThe video 'Mass Percent Made Easy' by Ketzbook teaches viewers how to calculate mass percent concentration and determine the mass of solute or solvent in a solution. It explains mass percent as a measure of solute per 100 units of solution, using the equation (mass of solute / total mass of solution) * 100. Examples include calculating the mass percent of sodium bromide in an aqueous solution and determining the mass of solute in a toothpaste tube. The video also guides on making a 7.8% aqueous glucose solution using a given mass of glucose, emphasizing the practicality of weighing solvent separately. It concludes with instructions on preparing the solution and encourages engagement with the content.
Takeaways
- 😀 Mass percent is a concentration measurement representing the amount of solute in 100 units of solution.
- 📚 The equation for mass percent is (mass of solute / total mass of solution) * 100.
- 🧪 An example of mass percent is 2% milk, which means 2 grams of fat per 100 grams of milk.
- 🌡️ When calculating mass percent, units on the numerator and denominator must be the same to cancel out, leaving a dimensionless ratio.
- 💧 In an aqueous solution, water is the solvent, and for most calculations, 1 mL of water is approximately equal to 1 gram at room temperature.
- 🔢 To find the mass percent concentration, divide the mass of the solute by the total mass of the solution and multiply by 100.
- 🧴 For the toothpaste example, knowing the mass percent allows us to calculate the mass of sodium fluoride in the tube.
- 🍬 When preparing a solution, it's practical to weigh the solvent separately and then add the known mass of solute.
- 📉 If the goal is to find the mass of the solute, rearrange the mass percent equation to solve for the unknown.
- 🍯 The video provides a step-by-step guide on how to calculate mass percent and solve related problems, such as making a 7.8% aqueous glucose solution.
- 👍 The video concludes with instructions on how to combine the calculated amounts of solute and solvent to create the desired solution.
Q & A
What is mass percent and how is it defined?
-Mass percent is a way to measure concentration, defined as the amount of solute dissolved in 100 units of solution. It is expressed as a percentage where 'per' means for each and 'cent' is Latin for 100.
How is the mass percent of a solution calculated?
-The mass percent of a solution is calculated using the formula: mass percent = (mass of solute / total mass of the solution) × 100.
What does 'aqueous' mean in the context of a solution?
-In the context of a solution, 'aqueous' refers to a solution where water is the solvent.
What is the mass percent concentration of a solution with 1.96 grams of sodium bromide in 38.5 grams of water?
-The mass percent concentration of the solution is 5.09%, calculated by dividing 1.96 grams by 38.5 grams and multiplying by 100.
How can you determine the mass percent of fructose in a solution if you know the volume of water and the mass of fructose?
-First, convert the volume of water to mass using the density of water (approximately 1 g/mL at room temperature). Then, add the mass of fructose to the mass of water to get the total mass of the solution. Finally, calculate the mass percent by dividing the mass of fructose by the total mass of the solution and multiplying by 100.
How much sodium fluoride is in a 221 g tube of toothpaste that has a mass percent concentration of 0.24%?
-To find the mass of sodium fluoride, multiply the total mass of the toothpaste (221 g) by the mass percent concentration (0.24%) and divide by 100, which gives 0.53 grams.
What is the significance of the density of water when calculating mass percent in an aqueous solution?
-The density of water is significant because it allows us to convert the volume of water (the solvent) into mass. Since the density of water is approximately 1 g/mL at room temperature, we can directly convert mL to grams for the calculation.
How can you prepare a 7.8% aqueous solution of glucose using 5.0 g of glucose?
-To prepare a 7.8% solution, first calculate the total mass of the solution by dividing the mass of glucose (5 g) by the mass percent (7.8%) and multiplying by 100. This gives the total mass of the solution. Then, subtract the mass of glucose from the total mass to find the mass of water needed.
Why is it important to consider the mass of the solvent when making a solution?
-It is important to consider the mass of the solvent because the total mass of the solution is the sum of the mass of the solute and the mass of the solvent. Knowing the mass of the solvent helps in accurately preparing a solution with a specific mass percent concentration.
What is the final step in preparing an aqueous solution after calculating the necessary masses?
-The final step is to weigh the solute and solvent accurately, combine them in a beaker or flask, and stir until the solute is completely dissolved.
Why is caution advised when allowing children to use fluoride-containing toothpaste?
-Caution is advised because the amount of sodium fluoride in toothpaste, although small, can be dangerous for small children if ingested in large quantities, as it is approximately one-tenth the lethal dose for adults.
Outlines
🧪 Understanding Mass Percent and Calculations
This paragraph introduces the concept of mass percent as a method to measure the concentration of a solute in a solution. It explains that mass percent is the amount of solute per 100 units of solution and provides an example with milk's fat content. The main formula to calculate mass percent is given as (mass of solute / total mass of solution) * 100. The paragraph then walks through examples, including calculating the mass percent of a sodium bromide solution and determining the mass percent of fructose in water. It also covers how to find the mass of the solute when given the mass percent, using toothpaste containing sodium fluoride as an example. Key points include the cancellation of units in such calculations and the practical approach to solving for different components of a solution.
🍯 Preparing an Aqueous Glucose Solution with Specific Mass Percent
The second paragraph focuses on the practical application of mass percent calculations in preparing a solution. It discusses the process of making a 7.8% aqueous glucose solution starting with 5.0 grams of glucose. The paragraph outlines the steps to determine the total mass of the solution and then isolates the mass of the solvent needed. It emphasizes the importance of precise measurements in a lab setting and provides a clear method for calculating the required amounts of solute and solvent. The summary also suggests the best practices for measuring ingredients, such as weighing them for accuracy, and describes the final steps to obtain a homogeneous solution. The paragraph concludes with an invitation for feedback and further engagement through the provided website.
Mindmap
Keywords
💡Mass Percent
💡Solute
💡Solvent
💡Aqueous Solution
💡Concentration
💡Density
💡Significant Figures
💡Dimensionless Ratio
💡Toothpaste
💡Glucose
💡Beaker or Flask
Highlights
Introduction to the concept of mass percent and its calculation.
Definition of mass percent and its relation to the amount of solute in 100 units of solution.
Explanation of mass percent in the context of fat content in milk as an example.
Presentation of the equation for calculating mass percent.
Example calculation of mass percent concentration of a sodium bromide solution.
Clarification on the term 'aqueous' and its significance in solution composition.
Demonstration of how to calculate mass percent using a calculator.
Explanation of units cancellation in mass percent calculations.
Example calculation involving volume and mass of water as a solvent.
Use of water's density to convert volume to mass for calculations.
Guidance on calculating mass percent when the mass of the solution is unknown.
Method to solve for the mass of the solute given the mass percent and total mass of the solution.
Process of rearranging the mass percent equation to solve for unknown quantities.
Safety warning regarding the toxicity of sodium fluoride.
Step-by-step guide to creating a specific mass percent solution using a known mass of solute.
Practical advice on measuring solvent for solution preparation in a laboratory setting.
Final instructions for combining solute and solvent to create the desired solution.
Transcripts
Browse More Related Video

Calculate %m/m (Percent by Mass of a solution)

How to calculate percent concentration | Percent mass | Percent volume | Percent mass-volume - Dr K

How to Calculate Mass Percent of a Solution

Calculate %m/v, Mass-Volume Percent + 2 Examples
The Percentages You'll See in Chemistry
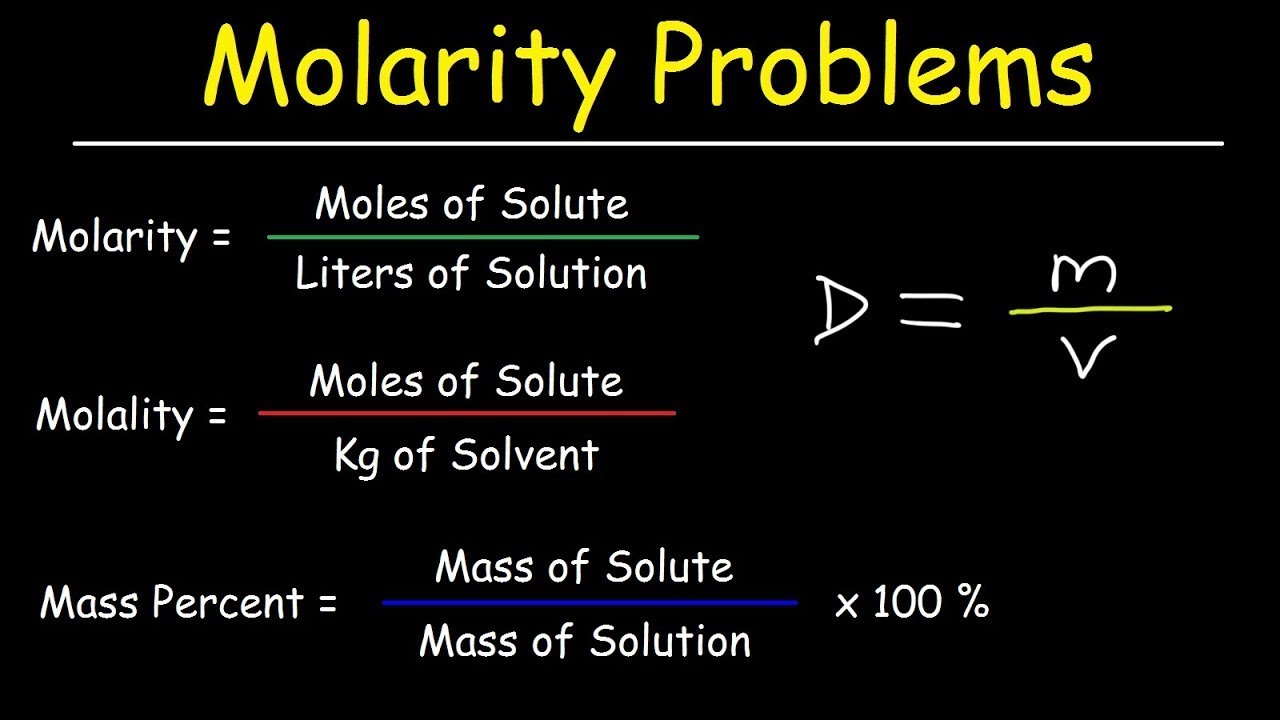
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration Problems
5.0 / 5 (0 votes)
Thanks for rating: