Atomic Structure: Protons, Electrons & Neutrons
TLDRThis educational video script explores the fundamental building blocks of atoms, highlighting the roles of protons, neutrons, and electrons. It introduces the concept of atomic number and how it correlates with the number of protons, which determines an element's identity. The script simplifies the atomic structure by illustrating the electron configuration of the first ten elements, explaining how the arrangement of subatomic particles dictates their properties and behavior. The video aims to demystify the periodic table and the underlying atomic structure that shapes our world.
Takeaways
- 🌌 All atoms in the periodic table are made from three particles: protons, electrons, and neutrons.
- 🔴 Protons and neutrons reside in the nucleus at the center of an atom, with protons carrying a positive charge and neutrons being neutral.
- ⚫ Electrons orbit the nucleus, forming a fuzzy ball shape, and carry a negative charge.
- ⚖ The overall neutrality of atoms is due to the balance between the positive charge of protons and the negative charge of electrons.
- 💡 The simplest atom, hydrogen, has one proton and one electron, establishing the rule that the number of protons equals the number of electrons in an atom.
- 🚀 Neutrons are necessary in atomic nuclei with more than one proton to provide additional 'glue' and prevent repulsion between protons from tearing the nucleus apart.
- 💡 The strong nuclear force is a fundamental force that holds the nucleus together, a concept typically introduced at the university level.
- 🌀 Electrons are arranged in shells around the nucleus, with the first shell holding up to two electrons and subsequent shells holding more.
- 📊 The electron configuration, which describes the distribution of electrons across the shells, is crucial for determining an element's properties.
- 🔢 The atomic number of an element is equal to the number of protons in its nucleus, which defines the element's identity.
- 🔠 Electron configurations, such as 2,1 for lithium, are written with the number of electrons in each shell, separated by commas.
Q & A
What are the three fundamental particles that make up atoms?
-The three fundamental particles that make up atoms are protons, electrons, and neutrons.
Where do protons and neutrons reside within an atom?
-Protons and neutrons reside in the atom's center, known as the nucleus.
What is the charge of a proton and how does it compare to a neutron?
-A proton carries a positive electric charge, while a neutron is neutral, carrying no charge.
What is the role of electrons in an atom and what charge do they carry?
-Electrons orbit the nucleus and trace out a fuzzy ball shape. They carry a negative electric charge.
Why are most atoms electrically neutral?
-Atoms are electrically neutral because the positive charge of the protons is exactly canceled by the negative charge of the electrons.
What is the atomic number of hydrogen and how is it constructed?
-Hydrogen has an atomic number of one and is constructed with one proton and one electron.
Why do all atoms need neutrons in their nucleus except hydrogen?
-All atoms except hydrogen need neutrons in their nucleus to provide additional 'glue' and counteract the repulsive forces between positively charged protons.
What is the electron configuration of lithium and how does it differ from hydrogen?
-Lithium has an electron configuration of 2,1, meaning it has two electrons in the first shell and one in the second shell, differing from hydrogen which has only one electron.
What is the significance of the first electron shell's capacity?
-The first electron shell can only hold two electrons before it becomes full, which is why elements with more than two electrons have additional shells.
What is the electron configuration of beryllium and how does it relate to its atomic number?
-Beryllium has an electron configuration of 2,2, which corresponds to its atomic number of four, indicating four protons and four electrons.
Why is the atomic number of an atom defined by the number of protons rather than electrons?
-The atomic number is defined by the number of protons because their number remains constant within an atom, whereas electrons can be gained or lost, altering the electron count.
What is the electron configuration of carbon and how does it influence its properties?
-Carbon has an electron configuration of 2,4. This configuration, with four electrons in its outer shell, influences its ability to form a variety of chemical bonds, making it a fundamental element in organic chemistry.
Why is neon considered a noble gas and unreactive?
-Neon is considered a noble gas and unreactive because its second shell is full with eight electrons, creating a stable electron configuration that does not readily participate in chemical reactions.
Outlines
🌌 Basic Structure of Atoms
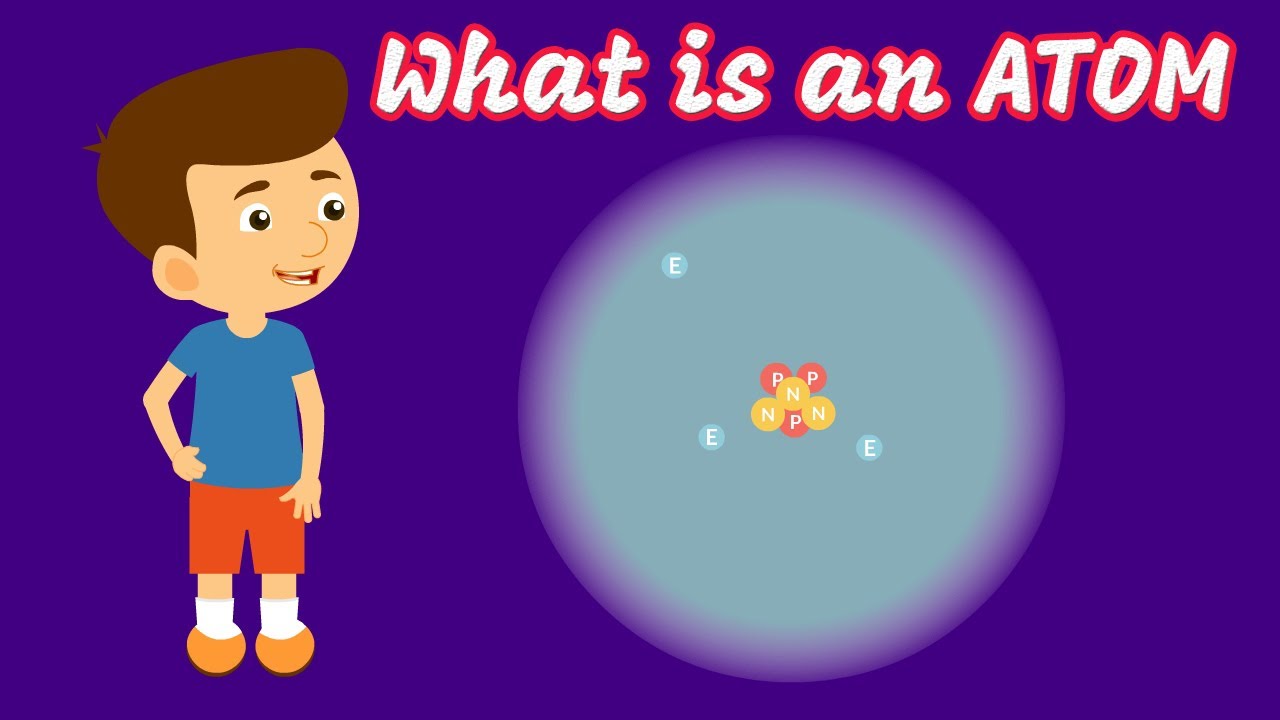
This paragraph introduces the fundamental components of atoms: protons, neutrons, and electrons. It explains that protons and neutrons, which are heavier, reside in the nucleus, with protons carrying a positive charge and neutrons being neutral. Electrons, lighter in mass, orbit the nucleus and carry a negative charge. The paragraph also discusses the concept of atoms being electrically neutral, meaning the number of protons and electrons must be equal. The simplest atom, hydrogen, is highlighted as having one proton and one electron. The paragraph further explains the role of neutrons in stabilizing the nucleus through the strong nuclear force, which is not typically taught until university level.
🚀 Electron Shells and Atomic Structure
The second paragraph delves into the concept of electron shells, which are layers that surround the atomic nucleus and can accommodate a specific number of electrons. It explains that the first shell can hold up to two electrons, and subsequent shells can hold more, with the second shell able to hold up to eight. Using the example of lithium, the paragraph illustrates how atoms with more than two electrons have their extra electrons in higher shells. The electron configuration, which describes the number of electrons in each shell, is emphasized as crucial for determining an element's properties. The paragraph also introduces the terms 'metal' and 'noble gas' in relation to electron configurations and hints at further explanations to come.
🔬 Building the Periodic Table
This paragraph continues the exploration of atomic structure by constructing the first ten elements of the periodic table. It discusses the atomic number, which corresponds to the number of protons in the nucleus and is unique to each element, defining its identity. The paragraph explains that while the number of electrons can change, the number of protons remains constant, making it a more reliable indicator of an element's identity. The electron configurations for nitrogen, oxygen, fluorine, neon, and others are outlined, showing the pattern of electron distribution across shells. The paragraph concludes by emphasizing the significance of subatomic particles and their arrangement in determining the properties and behavior of elements in the world.
Mindmap
Keywords
💡Protons
💡Electrons
💡Neutrons
💡Nucleus
💡Atomic Number
💡Electron Shells
💡Electron Configuration
💡Strong Nuclear Force
💡Periodic Table
💡Chemical Properties
Highlights
All atoms in the periodic table are made from three kinds of particles: protons, electrons, and neutrons.
Protons and neutrons reside in the nucleus, with protons carrying a positive charge and neutrons being neutral.
Electrons orbit the nucleus, forming a fuzzy ball shape, and carry a negative charge.
Atoms are overall neutral, with the number of protons equaling the number of electrons.
Hydrogen, the simplest atom, has one proton and one electron.
Helium, the next atom on the periodic table, requires 2 protons and 2 electrons to maintain neutrality.
Neutrons provide 'extra glue' to hold the nucleus together against repulsive forces.
The strong nuclear force works between nuclear particles to keep the nucleus intact.
Hydrogen is unique in not requiring a neutron, as it has only one proton.
Electrons live in shells around the nucleus, with the first shell taking only two electrons before it becomes full.
Lithium has three protons and three electrons, with one electron in the second shell.
The electron configuration affects the properties of an element and its behavior.
Lithium is a metal due to its single electron in the outer shell.
Beryllium has an electron configuration of 2,2, with all four electrons filling the first two shells.
Boron has an electron configuration of 2,3, with five electrons distributed across two shells.
Carbon has six protons and six electrons with an electron configuration of 2,4.
The atomic number of an atom is always the same as the number of protons in its nucleus.
Neon has a full second shell with eight electrons, making it an unreactive noble gas.
The electron configuration of an atom is crucial for understanding its chemical properties.
The world is shaped by the arrangement of these subatomic particles and their atomic structure.
Transcripts
Browse More Related Video

Inside Atoms: The Proton Numbers
What is an Atom? - Structure of an Atom - Atom video for kids

What Is An Atom - Part 1 | Properties of Matter | Chemistry | FuseSchool
Elements and atoms | Atoms, compounds, and ions | Chemistry | Khan Academy

Atoms | What are They? What are Protons, Neutrons and Electrons?

How to find the number of protons, neutrons, and electrons from the periodic table
5.0 / 5 (0 votes)
Thanks for rating: