Atoms | What are They? What are Protons, Neutrons and Electrons?
TLDRThis educational video delves into the fundamentals of atomic structure, explaining what an atom is and its composition of neutrons, protons, and electrons. It highlights the significance of the atomic nucleus and electron shell, and how these relate to the element's position on the periodic table. The video also covers how to calculate subatomic particles, emphasizing the properties of mass and charge, and uses carbon as an example to illustrate the concepts of atomic number and atomic weight. It concludes with a reminder of the importance of understanding these basics for anyone studying chemistry.
Takeaways
- 🌟 Atoms are the smallest particles of a chemical element that can exist, with a size of approximately 0.0000000001 cm.
- 🚀 An atom consists of three subatomic particles: neutrons, protons, and electrons.
- 🧬 The electron shell, represented by white lines, is where electrons orbit around the nucleus.
- 💫 The nucleus, the dense core at the center of an atom, contains protons and neutrons.
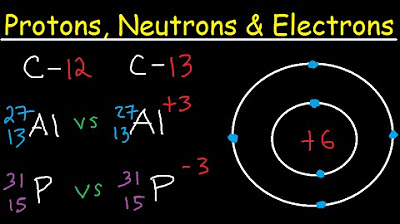
- 🔋 Protons have a positive charge and a mass of 1 atomic mass unit (amu), while neutrons are neutral with the same mass.
- ⚡️ Electrons have a negative charge, almost negligible mass, and orbit the nucleus in specific energy levels.
- 📊 The periodic table uses element symbols, atomic numbers, and atomic weights to represent elements.
- 🔢 The atomic number corresponds to the number of protons in an atom, which also equals the number of electrons in a neutral atom.
- 🤹♂️ The atomic weight (amu) of an element is the sum of the number of protons and neutrons.
- 🧩 To find the number of neutrons: mass number (atomic weight) minus the atomic number (number of protons).
- 🎓 Understanding the structure and properties of atoms is fundamental to grasping chemical reactions and the periodic table.
Q & A
What is an atom?
-An atom is the smallest particle of a chemical element that can exist, with a size approximately 0.0000000001 centimeters wide.
What are the three subatomic particles that make up an atom?
-The three subatomic particles that make up an atom are neutrons, protons, and electrons.
What is the function of the electron shell in an atom's structure?
-The electron shell is the region surrounding the nucleus where electrons are located and spread out in orbits.
What is the nucleus of an atom?
-The nucleus is the dense core at the center of the atom, containing protons and neutrons.
How can you remember the charges of protons, neutrons, and electrons?
-Protons have a positive charge (pro for positive), neutrons have a neutral charge (new for neutral), and electrons have a negative charge.
What does the atomic number represent?
-The atomic number represents the number of protons in an atom, which is also equal to the number of electrons in a neutral atom.
What is the atomic weight of an element?
-The atomic weight of an element is the sum of the number of protons and neutrons in its nucleus.
How can you calculate the mass number of an element?
-The mass number of an element can be calculated by adding the number of protons and the number of neutrons together.
How do you determine the number of neutrons in an atom if you only know the mass number?
-To determine the number of neutrons, subtract the atomic number (number of protons) from the mass number.
What are the two sets of numbers and a letter in the chemical symbol of an element?
-The letter is the element symbol, the top number is the atomic weight, and the bottom number is the atomic number.
Why are electrons not included in the calculation of atomic weight?
-Electrons are not included in the calculation of atomic weight because they have almost zero mass compared to protons and neutrons.
Outlines
🔬 Introduction to Atoms and Subatomic Particles
This paragraph introduces the concept of atoms as the smallest particles of a chemical element that can exist, with a size of approximately 0.0000000001 centimeters. It explains that atoms are composed of three subatomic particles: neutrons, protons, and electrons. The structure of an atom is described, with electrons orbiting in the electron shell around the nucleus, which contains protons and neutrons. The paragraph also touches on the calculation of subatomic particles, emphasizing the properties of mass and charge for each particle type.
📊 Understanding Atomic Structure and Calculation
The second paragraph delves deeper into the atomic structure, explaining the role of protons, neutrons, and electrons with their respective masses and charges. It introduces the method of interpreting the chemical symbol of an element, highlighting the atomic weight and atomic number. The paragraph also presents equations used to calculate the mass number, the number of neutrons, and the atomic number, reinforcing the concept that an atom has an equal number of protons and electrons.
Mindmap
Keywords
💡Atom
💡Subatomic Particles
💡Nucleus
💡Electron Shell
💡Atomic Number
💡Atomic Weight
💡Periodic Table
💡Chemical Elements
💡Chemical Formulas
💡Charge
💡Mass
Highlights
Atoms are the smallest particles of a chemical element that can exist.
An atom is about 0.0000000000001 centimeters wide.
A stack of atoms equivalent to the thickness of a crisp would equal the height of Mount Everest.
Atoms consist of three smaller particles: neutrons, protons, and electrons.
The electron shell is the area surrounding the nucleus where electrons orbit.
The nucleus is the dense core at the center of the atom, containing protons and neutrons.
Each subatomic particle has a mass and a charge.
Protons have a positive charge and neutrons are neutral.
Electrons have almost zero mass and carry a negative charge.
The charge mnemonic: 'Pro' for positive, 'Ne' for neutral, 'Electron' for negative.
The periodic table uses element symbols and atomic numbers to represent elements.
The atomic number indicates the number of protons in an atom.
The atomic weight is the sum of the protons' and neutrons' masses.
Electrons' negligible weight is not included in the atomic weight calculation.
The mass number equals the sum of protons and neutrons.
The number of neutrons can be derived from the mass number minus the atomic number.
An atom always has the same amount of protons and electrons.
The chemical symbol is used in chemical formulas and equations.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: