Nuclear Fission
TLDRNuclear fission, the process of splitting unstable atoms like uranium-235 to release vast amounts of energy, is explained in this script. The explanation details how the addition of a neutron to uranium-235 results in the creation of uranium-236, which then splits into two more stable elements, releasing energy and additional neutrons. These neutrons can then trigger further fission in a chain reaction, which is harnessed in nuclear power plants and submarines, but controlled to prevent destructive explosions. The script also mentions that elements with atomic numbers between 90 and 100 are similarly unstable and can undergo fission.
Takeaways
- 🔬 Nuclear fission is the process of splitting an atom into two or more smaller pieces, releasing a significant amount of energy.
- ⚛️ The energy released in nuclear fission comes from the transition of an unstable atom to a more stable state.
- 🏠 Nuclear fission provides energy for applications such as nuclear submarines, nuclear power plants, and nuclear bombs.
- 📈 Uranium-235 is an unstable atom that can be made to split by adding a neutron, resulting in the formation of Uranium-236.
- 💥 Uranium-236 is highly unstable and splits into smaller atoms like Krypton-92 and Barium-141, along with the release of three neutrons.
- 🔄 The released neutrons can cause a chain reaction, where they split more atoms, leading to a rapid and significant release of energy.
- 🗝️ Not only Krypton and Barium, but other elements like Rubidium, Cesium, Strontium, and Xenon can also be produced in a nuclear fission reaction.
- 🔢 Most elements with atomic numbers from 90 to 100 are unstable enough to undergo fission when provoked, such as by adding neutrons.
- 🚦 The chain reaction in nuclear fission can be controlled by using smaller amounts of uranium or by employing neutron-absorbing compounds.
- 🌐 The rate of energy release in nuclear fission is akin to how rumors spread; one split can lead to many, creating a chain reaction of energy production.
Q & A
What is nuclear fission?
-Nuclear fission is a process in which an unstable atomic nucleus splits into two or more smaller and more stable nuclei, releasing a significant amount of energy in the process.
What kind of energy sources utilize nuclear fission?
-Nuclear fission is used as a power source in nuclear power plants, propels nuclear submarines, and can also be employed in the creation of nuclear bombs.
How does the process of nuclear fission start?
-Nuclear fission can be initiated by bombarding an unstable atom, such as uranium-235, with a neutron, causing it to become unstable and split.
What happens when uranium-235 undergoes fission?
-When uranium-235 absorbs a neutron, it becomes uranium-236, which is highly unstable and splits into two smaller nuclei, such as krypton-92 and barium-141, along with releasing additional neutrons.
Why are the products of fission more stable than the original nucleus?
-The products of fission are more stable because they have a more balanced ratio of protons to neutrons, which leads to a lower overall energy state, making them less likely to undergo further radioactive decay or fission.
What is a chain reaction in the context of nuclear fission?
-A chain reaction occurs when the neutrons released by one fission event go on to cause further fission in other unstable nuclei, creating a self-sustaining series of fission events that release a large amount of energy.
How can a chain reaction be controlled?
-A chain reaction can be controlled by using a smaller amount of fissile material to limit the number of neutrons available for further fission, or by introducing neutron-absorbing materials that prevent the neutrons from causing additional fission events.
What are the products of a uranium-235 fission event?
-In a typical uranium-235 fission event, the nucleus splits into smaller nuclei such as krypton-92 and barium-141, and also releases three neutrons that can potentially cause further fission.
Are there other elements besides uranium that can undergo fission?
-Yes, most elements with atomic numbers between 90 and 100 are unstable enough to undergo fission when provoked, such as by adding neutrons.
How does the process of nuclear fission relate to a chain reaction?
-The process of nuclear fission naturally leads to a chain reaction, as the neutrons released by each fission event can go on to cause further fission in other unstable nuclei, leading to an exponential increase in the number of fission events and the energy released.
What is the significance of the energy released in nuclear fission?
-The energy released in nuclear fission is tremendous and can be harnessed for various applications, from powering large-scale energy plants to providing propulsion for submarines, and unfortunately, also for creating destructive nuclear weapons.
Outlines
🔌 Understanding Nuclear Fission
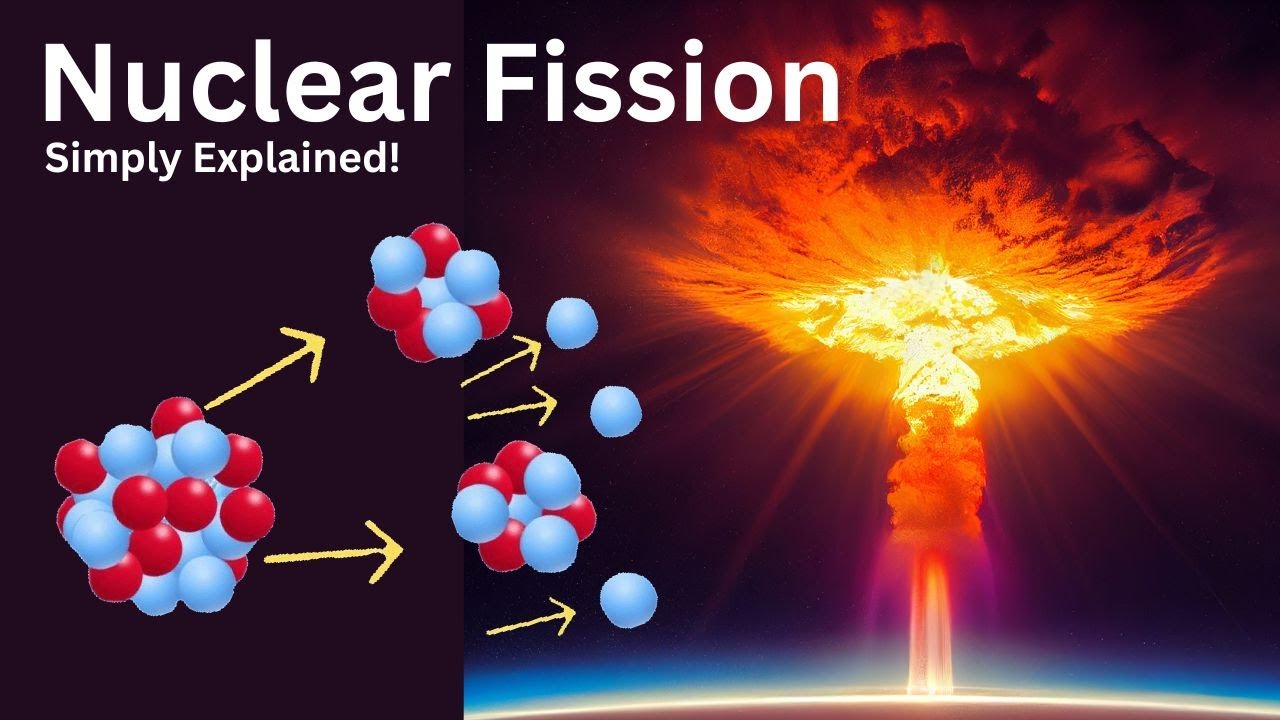
This paragraph introduces the concept of nuclear fission, a process often referred to as 'splitting the atom.' It explains that in nuclear fission, an unstable atom splits into two or more smaller pieces, releasing a significant amount of energy. This energy is harnessed for various applications, such as powering nuclear submarines, nuclear power plants, and even nuclear bombs. The paragraph describes the process starting with an unstable atom, like uranium-235, which absorbs a neutron and becomes uranium-236, leading to its split into more stable atoms like krypton-92 and barium-141. Additionally, the process releases three neutrons, which can further cause other atoms to split, creating a chain reaction. The paragraph emphasizes the transition from unstable to stable atoms as the source of energy in nuclear fission.
💥 The Chain Reaction of Nuclear Fission
This paragraph delves into the role of neutrons in sustaining a chain reaction during nuclear fission. It describes how the initial splitting of a uranium atom releases three neutrons, each of which can cause further atoms to split, leading to a rapid and extensive chain reaction. The paragraph uses an analogy of rumors spreading to explain the exponential nature of the chain reaction. It also discusses the potential for this chain reaction to release tremendous amounts of energy, as seen in nuclear bombs, and the methods to control the reaction for applications like nuclear power plants and submarines. The control mechanisms include using a smaller amount of uranium to limit the reaction or using compounds to absorb neutrons and prevent them from causing additional splits.
Mindmap
Keywords
💡Nuclear Fission
💡Unstable Atom
💡Neutron
💡Chain Reaction
💡Energy Release
💡Nuclear Reactor
💡Nuclear Bomb
💡Uranium-235
💡Krypton-92 and Barium-141
💡Control Rods
💡Fissile Material
Highlights
Nuclear fission is a process that involves splitting an atom into two or more smaller pieces, releasing a tremendous amount of energy.
The energy from nuclear fission is used in nuclear submarines, nuclear power plants, and even nuclear bombs.
Unstable atoms, also known as 'unhappy' atoms, undergo fission to become more stable, releasing energy in the process.
Uranium-235 is an example of an unstable atom that can undergo fission when hit with a neutron.
When uranium-235 absorbs a neutron, it becomes uranium-236, which is even more unstable and splits into smaller atoms.
The fission of uranium-236 typically results in the creation of krypton-92, barium-141, and the release of three neutrons.
The fission process can also produce different elements such as rubidium, cesium, strontium, and xenon.
Most elements with atomic numbers from 90 to 100 are unstable and can undergo fission.
The release of neutrons from one fission event can cause a chain reaction, leading to the splitting of multiple atoms.
A chain reaction can release a large amount of energy quickly, as seen in nuclear bombs.
In controlled environments like nuclear power plants, the chain reaction is managed to prevent uncontrolled energy release.
Controlling the amount of uranium or using neutron-absorbing compounds can regulate the fission process.
The fission process is likened to the spread of rumors, where one piece of information leads to more and more sharing.
The chain reaction in nuclear fission is a self-sustaining process, where each fission event triggers more fissions.
The practical applications of nuclear fission include energy production, but it must be carefully managed to avoid catastrophic events.
Understanding the behavior of unstable atoms and their fission process is crucial for harnessing nuclear energy safely and effectively.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: