Nuclear Half Life: Intro and Explanation
TLDRThe video script provides an insightful explanation of the concept of nuclear half-life, a fundamental concept in nuclear physics. It describes how half-life measures the time it takes for half of a given amount of a radioactive substance to decay into another element. Using thorium-234 as an example, the script illustrates how it undergoes beta decay to become protactinium, with a half-life of 24 days. The script also compares this to other elements like uranium-238, which has a half-life of 4.5 billion years, and polonium-218, with a half-life of just three minutes. The explanation is complemented by a visual representation of the decay process over time, emphasizing the wide variation in half-lives among different elements. The script concludes with an invitation to engage in calculations related to half-life, appealing to both visual and mathematical learners.
Takeaways
- 🔬 **Nuclear Half-Life Definition**: The time it takes for half of a given amount of a radioactive substance to decay into another element.
- ⏳ **Example of Thorium Decay**: Thorium-234 undergoes beta decay to become protactinium, with a half-life of 24 days.
- 📉 **Decay Process**: Over time, the amount of the original substance (thorium) decreases as it transforms into a new element (protactinium).
- 🧬 **Change in Atomic Nucleus**: A nuclear process involves a change in the number of protons and/or neutrons within an atom's nucleus, leading to a different element.
- ⚖️ **Element Identity**: The number of protons in an atom's nucleus determines the element's identity, thus a change in proton number results in a new element.
- 📈 **Half-Life Graphical Representation**: A graph can illustrate the decay process, showing the remaining amount of the substance after each half-life period.
- 🕰️ **Variation in Half-Lives**: Different elements have different half-lives, ranging from very short (e.g., polonium-218 with a half-life of minutes) to very long (e.g., uranium-238 with a half-life of billions of years).
- 📊 **Half-Life Calculation**: The half-life can be calculated by observing the amount of substance remaining after a known period and determining the time taken for half of it to decay.
- ⏯️ **Continuous Decay**: The decay process is continuous, not a one-time event, and follows a predictable pattern based on the half-life of the substance.
- 🔴 **Visualizing Decay**: Visual aids, such as graphs, can help understand the concept of half-life by showing the progressive reduction in the amount of the substance over time.
- ⚠️ **Implications of Decay**: Understanding half-life is crucial for various applications, including dating ancient materials, medical treatments, and managing nuclear waste.
Q & A
What is a nuclear process?
-A nuclear process is a change in the number of protons and neutrons in an atom's nucleus.
What happens during beta decay?
-During beta decay, one of the neutrons in an atom's nucleus turns into a proton.
How does the number of protons in an atom affect its identity?
-The number of protons in an atom determines the type of element it is. A change in the number of protons results in a different element.
What is an example of a nuclear decay process described in the script?
-The script describes the decay process of thorium-234 turning into protactinium through beta decay.
What is meant by the term 'half-life' in the context of nuclear decay?
-Half-life is the time required for half of a given amount of a radioactive substance to decay into a different element.
What is the half-life of thorium-234 in the example provided?
-The half-life of thorium-234 in the example is 24 days.
How does the amount of thorium change after each half-life in the example?
-After each half-life, the amount of thorium is reduced by half, as the other half has decayed into protactinium.
What does the abbreviation T_1/2 represent?
-T_1/2 is the abbreviation for half-life.
What is the half-life of uranium-238 in comparison to thorium-234?
-The half-life of uranium-238 is 4.5 billion years, which is significantly longer than the half-life of thorium-234.
What is the half-life of polonium-218 and how does it compare to the half-life of thorium-234?
-The half-life of polonium-218 is only three minutes, which is much shorter than the half-life of thorium-234.
How do different decay processes vary in terms of their half-lives?
-Different decay processes have widely varying half-lives, from as short as a few minutes to as long as billions of years.
What is the significance of understanding half-life in the study of nuclear reactions?
-Understanding half-life is crucial for predicting how quickly a radioactive substance will decay, which is important for various applications such as dating geological samples, medical treatments, and safety considerations.
Outlines
🔬 Understanding Nuclear Half-Life
The first paragraph introduces the concept of nuclear half-life and its significance in nuclear processes. It explains that a nuclear process involves a change in the number of protons and neutrons in an atom's nucleus, using the example of thorium-234 undergoing beta decay to become protactinium. The half-life is defined as the time it takes for half of a given amount of a substance to decay. The example of thorium-234 is used to illustrate this, where starting with 80 grams, half of it decays to protactinium every 24 days. The paragraph also emphasizes that the term 'disappear' refers to the substance changing into a different element, not vanishing into nothingness. The half-life is represented by T_1/2 and for thorium-234, it is 24 days. A visual representation of the decay process is mentioned, showing the halving of the substance's amount after each half-life period.
⏳ Variability in Half-Life Periods
The second paragraph discusses the variability in half-life periods among different elements. It contrasts the long half-life of uranium-238, which is 4.5 billion years, with the very short half-life of polonium-218, which is just three minutes. The paragraph illustrates the practical implications of these differences by humorously suggesting that half of a polonium-218 sample could decay into lead while someone is away from the lab for a short break. It emphasizes that the time between each halving of the sample's amount (each half-life period) varies widely for different elements, and this is a key characteristic of their radioactive decay processes.
Mindmap
Keywords
💡Nuclear Half-life
💡Nuclear Process
💡Beta Decay
💡Protons and Neutrons
💡Element
💡Thorium-234
💡Protactinium
💡Half-Life Calculation
💡Radioactive Decay
💡Uranium-238
💡Polonium-218
Highlights
Nuclear half-life is a measure of the time it takes for nuclear processes to occur, where the number of protons and neutrons in an atom's nucleus change.
An example of a nuclear process is the beta decay of thorium-234, where a neutron transforms into a proton, altering the element from thorium to protactinium.
The number of protons in an atom determines the element's identity, and a change in this number results in a different element.
Starting with 80 grams of thorium, after 24 days, half of it decays to protactinium, leaving 40 grams of thorium.
The half-life of thorium-234 is 24 days, meaning every 24 days the amount of thorium reduces by half as it decays into protactinium.
The term 'half-life' is often abbreviated as T1/2, and for thorium-234, T1/2 is 24 days.
A visual representation of half-life can be seen in a graph where the amount of a substance decreases by half after each half-life period.
Different decay processes have varying half-lives, from billions of years for uranium-238 to just minutes for polonium-218.
Uranium-238 undergoes alpha decay to form thorium-234 with a half-life of 4.5 billion years.
Polonium-218 has a much shorter half-life of three minutes, undergoing alpha decay to become lead-214.
The concept of half-life is crucial for understanding the rate at which radioactive substances decay and transform into other elements.
The half-life is the time required for half of a sample to decay, which can be visualized as a stepwise decrease over time.
The transformation of one element to another through decay is not about disappearance but a change in the atomic structure.
The half-life concept is fundamental in nuclear physics and has practical applications in dating, energy production, and medical treatments.
The half-life can be used to calculate the remaining amount of a radioactive substance after a given period.
Understanding the half-life of various elements is essential for safe handling and disposal of radioactive materials.
The half-life is a key parameter in the field of nuclear energy, influencing the management of nuclear fuel and waste.
The study of half-life is also important in archaeological and geological dating methods, such as radiocarbon dating.
Transcripts
Browse More Related Video

Half-life and carbon dating | Nuclear chemistry | Chemistry | Khan Academy
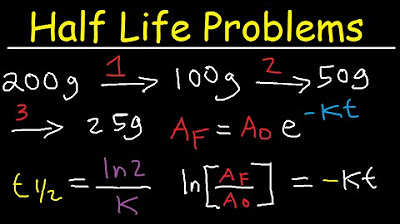
Half Life Chemistry Problems - Nuclear Radioactive Decay Calculations Practice Examples

Half-Life and Radioactive Decay

Exponential decay formula proof (can skip, involves calculus) | Chemistry | Khan Academy

Introduction to exponential decay | Nuclear chemistry | Chemistry | Khan Academy

15.4 Kinetics of Nuclear Decay | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: