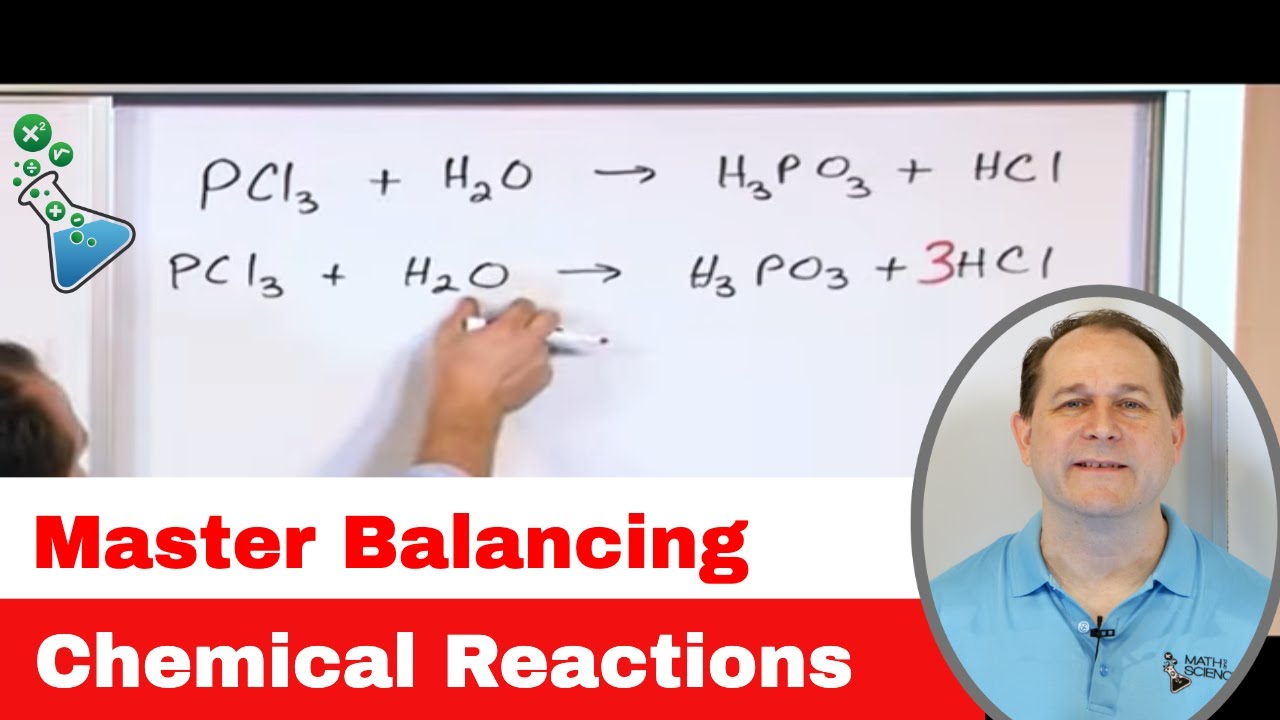
Good Thinking! — Chemical Reactions in Action
TLDRThe video script presents a dynamic chemistry lesson where students learn about chemical reactions through engaging demonstrations and discussions. The teacher uses everyday examples, like rust formation on a bike, to explain reactants and products. The lesson delves into atomic and molecular concepts, using marshmallows as a visual aid to illustrate how atoms combine to form new substances. The script also addresses common misconceptions about chemical reactions, emphasizing their reversibility and the importance of clearly defining the system under consideration.
Takeaways
- 🧪 Chemical reactions involve reactants forming new products through the rearrangement of atoms.
- 🚴♂️ Rusting is a chemical reaction where iron atoms in metal react with oxygen molecules from the air.
- 🎓 Students often benefit from visual models to understand abstract concepts like chemical reactions.
- 🍫 Using concrete objects like marshmallows can help illustrate how atoms combine to form molecules in chemical reactions.
- 🏫 Teaching the concept of a substance is important to explain what makes up materials like the iron in a bicycle.
- 🤔 Students should be encouraged to think on the molecular level to better understand chemical interactions.
- 📐 Physical models, while helpful, have limitations and should be complemented with other teaching tools.
- 🔍 Clearly defining the system under consideration can help avoid confusion in understanding chemical reactions.
- 💡 Chemical reactions are not always irreversible; many have a balancing point rather than an endpoint.
- 🌊 Analogies like the beach scenario can help students visualize dynamic equilibrium in chemical reactions.
- 🔥 Misconceptions about the irreversibility of chemical reactions should be addressed to enhance understanding.
Q & A
What is the main concept being taught in the classroom scenario?
-The main concept being taught is chemical reactions, specifically how substances (reactants) interact to form new substances (products).
What is the non-scientific reason given for the bike's brown color?
-The non-scientific reason provided is that the teacher had to start using a hand-me-down after their last bike was run over by a plantain truck.
How does the teacher describe the process of rust formation on the bike?
-The teacher describes the rust formation as a chemical reaction where iron atoms in the metal combine with oxygen molecules from the air to produce rust.
What is Amar's initial misunderstanding about the source of oxygen atoms in rust?
-Amar initially does not specify the source of oxygen atoms, implying that he might have thought they came from within the bike itself rather than the surrounding air.
How does the teacher use marshmallows to illustrate chemical reactions?
-The teacher uses brown marshmallows to represent iron atoms and red pairs to represent oxygen molecules, demonstrating through this physical model how the atoms combine to form rust.
What is the limitation of using a straight line arrangement of atoms to represent a rust molecule?
-The limitation is that rust molecules are not arranged in a straight line like a falafel kebab, so a physical model with atoms in a straight line might be misleading regarding the actual geometric arrangement of atoms in a molecule.
What misconception does the teacher address regarding the irreversibility of chemical reactions?
-The teacher addresses the misconception that chemical reactions are always irreversible. They explain that many reactions have a balancing point rather than being completely irreversible.
How does the teacher use the beach analogy to explain the concept of a balancing point in chemical reactions?
-The teacher uses the beach analogy where people are either on the sand or in the water, and there's a constant flux of people moving between the two states, creating a stable overall picture. This illustrates that chemical reactions also have a dynamic balance rather than a fixed endpoint.
What does the teacher suggest to help students understand the concept of a substance?
-The teacher suggests explaining what makes up a substance, such as explaining that the metal producing rust contains iron, which is made up of iron atoms.
What advice is given to teachers regarding the use of models in teaching chemical reactions?
-The advice given is to use a variety of models, pointing out their strengths and weaknesses, and reminding students that all models are imperfect representations of the actual processes.
Where can one find more information about how kids learn science and common misconceptions?
-More information can be found online at ScienceEducation.SI.edu/GoodThinking.
Outlines
🧪 Chemistry Lesson: Understanding Chemical Reactions
The paragraph introduces a chemistry lesson where the teacher uses humor and everyday examples to explain chemical reactions. The lesson begins with a humorous attempt at creating a dramatic explosion, but instead, the teacher uses the reaction between substances in a beaker and a test tube to illustrate how reactants transform into products. The teacher then uses the rusting of a bicycle as a real-life example of a chemical reaction, highlighting the importance of understanding atomic and molecular interactions. The paragraph emphasizes the need for clear explanations and the use of visual models to help students grasp abstract concepts in chemistry.
🎓 Teaching Strategies: Visual Models and Misconceptions
This paragraph discusses the teaching strategies for explaining chemical interactions on a molecular level. It emphasizes the use of visual models, such as marshmallows and food coloring, to help students understand how atoms rearrange to form different molecules. The paragraph also addresses common misconceptions about chemical reactions, such as the belief that they are always irreversible. The teacher and a colleague named Bunsen exchange ideas on how to effectively communicate the dynamic nature of reactions and the importance of defining the system under consideration. The conversation touches on the limitations of physical models and the need to challenge incorrect rules that have been traditionally taught.
🌐 Further Resources on Science Education
The final paragraph provides a resource for those interested in learning more about science education and the misconceptions that students may have. It directs the audience to an online platform, ScienceEducation.SI.edu/GoodThinking, where they can find additional information on how children learn science and the types of misconceptions that are common in the field. This paragraph serves as a call to action for educators and learners to seek out more comprehensive and accurate resources for understanding scientific concepts.
Mindmap
Keywords
💡Chemical Reaction
💡Reactants
💡Products
💡Atoms
💡Molecules
💡Rust
💡Conservation
💡Molecular Level
💡Physical Models
💡Misconceptions
💡Irreversibility
Highlights
Chemical reactions involve the formation of new substances from reactants.
The new substances formed in a chemical reaction are called products.
Rust formation on a bike is a chemical reaction involving the metal and oxygen from the air.
Teaching chemical reactions using atoms and molecules can improve students' understanding.
The concept of a substance should be explained in terms of its atomic composition.
Iron atoms in rust react with oxygen molecules from the air to form the compound.
Visualizing chemical reactions with concrete models, such as marshmallows, can aid in understanding.
The law of conservation is reinforced through the use of concrete models in chemical education.
Physical models have limitations and should be used alongside other teaching methods.
Ball and stick models can help convey the geometric arrangement of atoms within molecules.
It's important to clearly define the system being considered in chemical reactions.
The misconception that chemical reactions are one-way and irreversible is a common issue.
Chemical reactions often have a balancing point rather than being completely irreversible.
The idea of 'going to completion' in reactions is often misunderstood by students.
An analogy of people on a beach illustrates the dynamic balance of reactions.
Teachers should be aware of and address common misconceptions in chemical education.
The difference between physical and chemical changes is often misrepresented.
For more information on teaching science and addressing misconceptions, visit ScienceEducation.SI.edu/GoodThinking.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: