Given pH & pOH, Solve for [H+] & [OH-] Practice Problems
TLDRThe video script offers a comprehensive guide on solving pH-related problems, using two examples to illustrate the process. The first example involves an acidic HCL solution with a pH of 2.3, where the script explains how to calculate the hydrogen ion concentration and the hydroxide ion concentration. The second example features a basic sodium hydroxide solution with a pH of 9.3, detailing the steps to find the hydrogen and hydroxide ion concentrations. The video emphasizes the use of pH and p formulas, inverse log functions, and the relationship between pH values, exponents, and ion concentrations to determine the acidity or basicity of solutions.
Takeaways
- 📚 The pH scale is used to determine the acidity or basicity of a solution, with lower pH values indicating higher acidity and higher values indicating basicity.
- 🔢 Given a pH value, one can calculate the hydrogen ion (H+) concentration using the formula: 10^(-pH) to find the concentration in moles per liter.
- 📈 The relationship between pH and pOH is defined by the equation: pH + pOH = 14, which holds true at 25°C and helps in understanding the balance of acidity and basicity in a solution.
- 🧪 Example 1: A solution of HCl with a pH of 2.3 is acidic. Using the formula, the H+ ion concentration is calculated to be 5.0 * 10^(-3) moles per liter.
- 🧬 Example 2: A basic solution of sodium hydroxide with a pH of 9.3 has a hydrogen ion concentration of 5 * 10^(-10) moles per liter and a hydroxide ion concentration of 2 * 10^(-5) moles per liter.
- 📊 The pH value is inversely related to the hydrogen ion concentration; as pH decreases, H+ concentration increases, and vice versa.
- 📝 The pOH value is calculated using the formula: 10^(-pOH), which gives the hydroxide ion (OH-) concentration in moles per liter.
- 🔄 To find the pOH from the pH, subtract the pH value from 14. For a basic solution, the pOH is calculated by subtracting the given pH from 14.
- 📚 Understanding the relationship between pH, pOH, and ion concentrations is crucial for solving problems in chemistry and related fields.
- 📈 The exponent in the hydrogen ion concentration formula corresponds to the pH value, providing a quick way to estimate the concentration.
- 🔧 The use of a scientific calculator with the '10^x' function is essential for these calculations, as it simplifies the process and reduces the risk of error.
- 📝 Practice and understanding of these calculations are important for academic success in chemistry-related courses and for professional tasks in scientific fields.
Q & A
What is the pH value of the first solution mentioned in the script?
-The pH value of the first solution is 2.3.
What does a pH value less than 7 indicate about a solution?
-A pH value less than 7 indicates that the solution is acidic.
What is the relationship between pH and pOH?
-The relationship between pH and pOH is given by the formula: pH + pOH = 14.
How can you calculate the hydrogen ion concentration from pH value?
-You can calculate the hydrogen ion concentration using the formula: [H+] = 10^(-pH).
What is the pOH value of the first solution?
-The pOH value of the first solution is 11.7, calculated by subtracting the pH value from 14 (14 - 2.3).
What is the hydroxide ion concentration in the first solution?
-The hydroxide ion concentration in the first solution is 2.0 * 10^-12 Molar.
What is the pH value of the second solution discussed in the script?
-The pH value of the second solution is 9.3.
What does a pH value greater than 7 indicate about a solution?
-A pH value greater than 7 indicates that the solution is basic.
How can you calculate the hydroxide ion concentration from pOH value?
-You can calculate the hydroxide ion concentration using the formula: [OH-] = 10^(-pOH).
What is the hydrogen ion concentration in the second solution?
-The hydrogen ion concentration in the second solution is 5 * 10^-10 Molar.
What is the significance of the exponent in the hydrogen ion concentration in relation to pH?
-The exponent in the hydrogen ion concentration is very close to the pH value, which can be used as a double check for the calculations.
How can you verify your calculations for hydroxide ion concentration?
-You can verify your calculations by noting that the p number is close to the exponent of the hydroxide concentration, as seen in the second solution where the pOH is 4.7 and the hydroxide concentration is 2 * 10^5 Molar.
Outlines
📚 Understanding pH and pOH Calculations
This paragraph introduces the concept of pH and pOH calculations, using a specific example of a hydrochloric acid (HCl) solution with a pH of 2.3. The speaker explains the relationship between pH and pOH through the formula pH + pOH = 14, and demonstrates how to calculate the hydrogen ion (H+) concentration and hydroxide ion (OH-) concentration from the given pH value. The process involves using the inverse log function to find the concentration of H+ ions and rearranging formulas to solve for OH- concentration. The example concludes with the calculated concentrations of 5.0 * 10^-3 moles per liter for H+ ions and 2.0 * 10^-12 moles per liter for OH- ions, emphasizing the acidic nature of the solution.
🧪 Applying pH Calculations to a Basic Solution
The second paragraph focuses on applying pH calculations to a basic solution, specifically a sodium hydroxide (NaOH) solution with a pH of 9.3. The speaker guides the audience to understand that a basic solution dissociates to produce hydroxide ions (OH-) and uses the same formula pH + pOH = 14 to find the pOH value, which is 4.7 in this case. The explanation continues with the calculation of the hydrogen ion concentration using the inverse log function, resulting in a concentration of 5 * 10^-11 moles per liter. The speaker then rearranges the formula to calculate the hydroxide ion concentration, which is found to be 2 * 10^-5 moles per liter. The paragraph emphasizes the relationship between the pH value and the exponent of the hydroxide ion concentration, reinforcing the understanding of basic solutions.
Mindmap
Keywords
💡pH
💡Hydrogen ion concentration
💡Hydroxide ion concentration
💡Acidic solution
💡Basic solution
💡pOH
💡Inverse log function
💡Concentration
💡Molarity
💡Neutralization reactions
💡Chemical calculations
Highlights
The video provides examples of solving problems with given pH values.
The first example involves a solution of HCL with a pH of 2.3.
The pH value of 2.3 indicates a strong acidic solution.
The relationship between pH and pOH is explained using the formula pH + pOH = 14.
The calculation of pOH from pH is demonstrated, resulting in pOH = 11.7 for the HCL solution.
The hydrogen ion concentration is calculated using 10^(-pH), resulting in 5.0 * 10^-3 molar for the HCL solution.
The hydroxide ion concentration is found using 10^(-pOH), resulting in 2.0 * 10^-12 molar for the HCL solution.
The second example features a basic solution of sodium hydroxide with a pH of 9.3.
The pH of 9.3 confirms the basic nature of the sodium hydroxide solution.
The calculation of hydrogen ion concentration for the basic solution is shown, resulting in 5 * 10^-10 molar.
The hydroxide ion concentration for the basic solution is calculated as 2 * 10^-5 molar.
The video emphasizes the practical application of these calculations for a test or quiz.
The method for solving problems with given pH values is clearly outlined in a step-by-step manner.
The video encourages viewers to practice these calculations on their own before revealing the answers.
The video concludes with a recap of the process and well wishes for the viewer's test.
The use of the inverse log function is crucial for calculating ion concentrations from pH values.
The video demonstrates the importance of understanding the relationship between pH, pOH, and ion concentrations.
Transcripts
Browse More Related Video

Calculate the pH of Acids and Bases Given the Concentration of a Solution
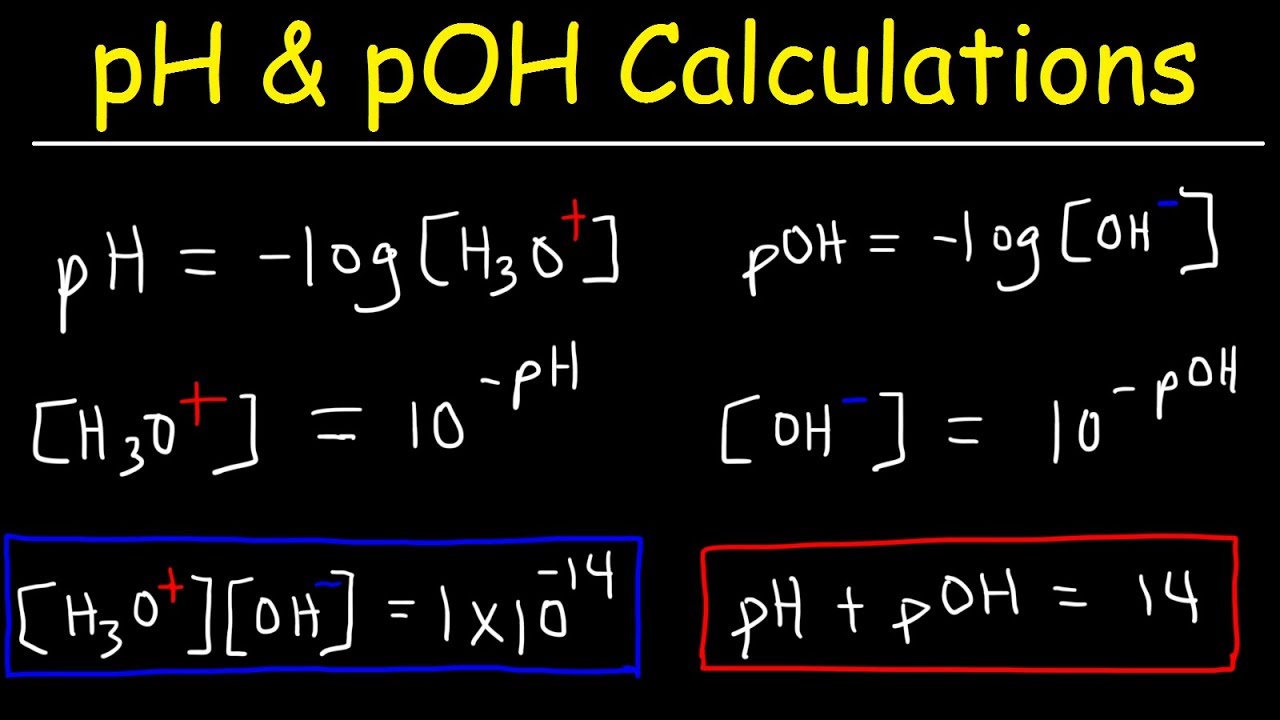
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
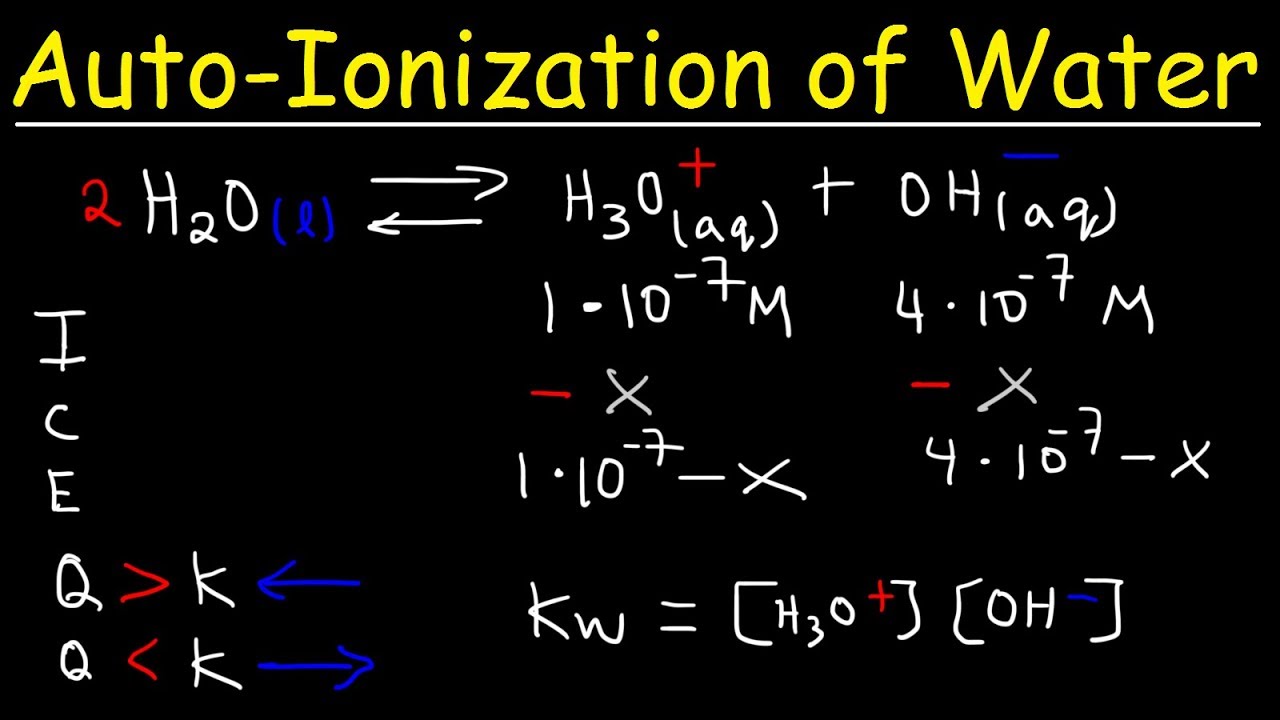
AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained
pH and pOH: Crash Course Chemistry #30

Determine pH from Kb and Base
5.0 / 5 (0 votes)
Thanks for rating: