CH403 5 Quality Assurance and Calibration Methods
TLDRThe transcript delves into the significance of quality assurance in laboratory practices, emphasizing its role in maintaining consistent and accurate results across different labs. It illustrates this with examples, such as the variation in results from different labs analyzing the same sample. The importance of precision and accuracy in measurements is highlighted, along with the concept of quality assurance as systematic monitoring and evaluation. The transcript also discusses the balance between the cost of testing and the potential loss from defects, using the analogy of spaghetti sauce production. It further explains the difference between raw data, treated data, and results, and the necessity of clear objectives and specifications for analytical procedures. The concept of selectivity and sensitivity in analysis is introduced, along with the impact of matrix effects on the accuracy of results. The transcript also touches on the importance of representative sampling and the definitions of selectivity, specificity, and sensitivity in analytical chemistry. It concludes with a discussion on standard addition and internal standard techniques for improving analysis accuracy and the use of efficient experimental design to minimize the number of required analyses.
Takeaways
- 🔍 Quality assurance is crucial for consistent results in lab analyses, as demonstrated by the variation in results from different labs when analyzing the same sample.
- 🌟 National Labs generally provide more accurate results within the certified range, showing the importance of quality assurance in maintaining high standards.
- 📈 The goal of quality assurance (QA) is to ensure that results meet customer needs and that the level of precision and accuracy aligns with the potential consequences of errors.
- 🥫 In production scenarios, such as manufacturing spaghetti sauce, QA involves sampling to ensure the batch meets quality standards without incurring unnecessary costs.
- 🧪 Chemists focus on raw data, treated data, and results, each requiring different levels of precision and accuracy based on the application.
- 📝 Clear and concise objectives for data and results are essential for establishing specifications and precautions necessary for the analytical procedure.
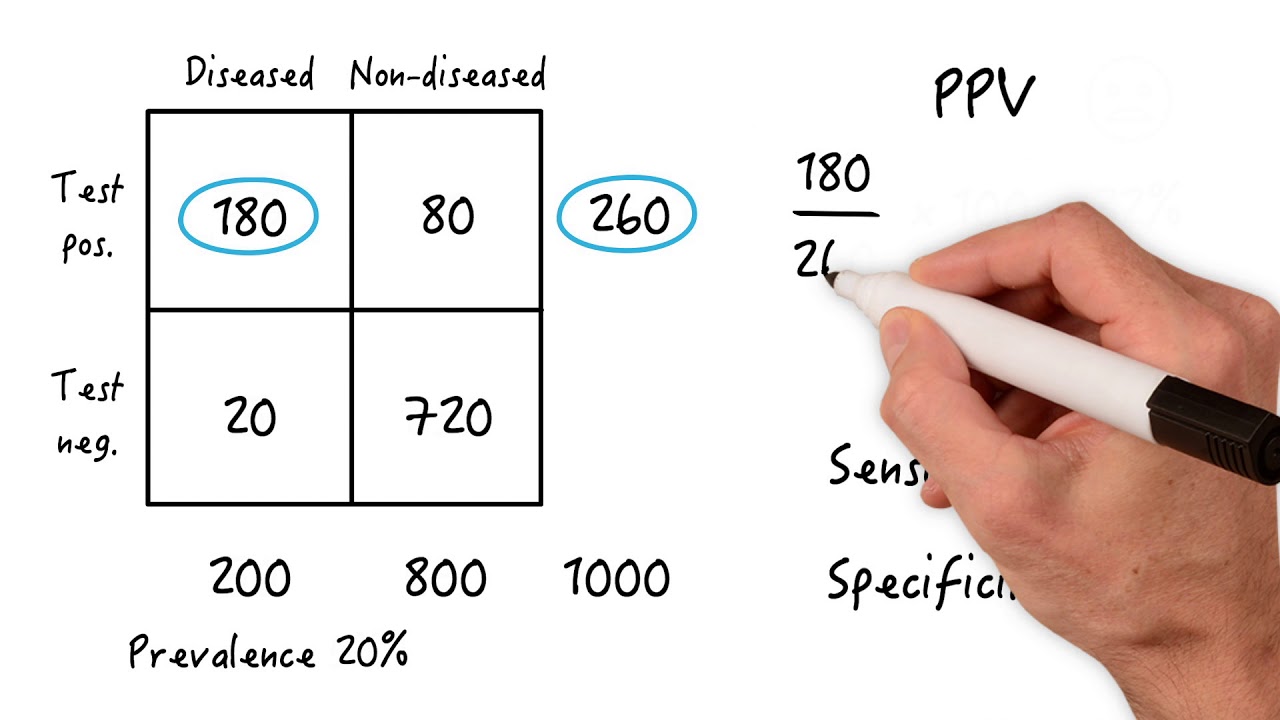
- 🔎 Selectivity (specificity) and sensitivity are key performance indicators in QA, ensuring accurate detection and reliable response to analyte concentration changes.
- 🌐 Representative sampling is vital for obtaining accurate data about a population, requiring knowledge of the sample's environment and potential variations within it.
- 📊 Standard addition and internal standard techniques are used to account for matrix effects and ensure accurate quantification of analytes in complex samples.
- 🧠 Efficient experimental design, such as fractional factorial design, can reduce the number of analyses needed to determine concentrations and uncertainties, leveraging computer analysis for optimization.
- 💼 Quality assurance and calibration methods are not only essential for scientific accuracy but also for the employability of chemists, with many well-paying jobs in this field.
Q & A
What is the main purpose of quality assurance in a project or facility?
-The main purpose of quality assurance is to systematically monitor and evaluate aspects of a project, facility, or service to ensure that standards of quality are met.
Why is it important to perform analysis in the same manner every time?
-It is important to perform analysis in the same manner every time to minimize variation in results and ensure that the measurements are as precise and accurate as possible, thereby getting values that are very close to the true or accepted value.
What is the difference between National Labs and other accredited labs in terms of results variation?
-National Labs tend to have results within the confidence interval that is within the certified range, while other accredited labs may show a huge variation in results, with some values being far too low or high compared to the certified range.
How does the example of making spaghetti sauce in a factory illustrate the concept of quality assurance?
-The spaghetti sauce example shows that in a factory setting, you cannot taste each jar, so you select a few jars for testing. If those few are okay, you assume the rest are also okay. However, if the number of bad jars is low, it may not be worth testing more, and quality assurance measures can help ensure that the results meet customer needs without excessive testing and waste.
What are the differences in quality assurance requirements for pharmaceuticals compared to spaghetti sauce?
-Pharmaceuticals require far more careful quality assurance because the consequences of errors can be significantly harmful, whereas with spaghetti sauce, the worst consequence might be a bad taste or the need for a refund.
What are raw data, treated data, and results in the context of quality assurance?
-Raw data are the individual measured quantities, such as volume from a test. Treated data are derived from raw data after applying a calibration procedure. Results are the final outputs, such as mean, standard deviation, and confidence interval, that are reported.
Why is it important to write clear and concise objectives for data and results in quality assurance?
-Clear and concise objectives help define the required level of accuracy and precision for the analytical procedure and the precautions needed to ensure that the results meet the specified quality standards.
What is the significance of selectivity (or specificity) in quality assurance?
-Selectivity is the ability to distinguish the analyte from other species in the sample, avoiding interference from unwanted substances. The more selective an analysis is, the fewer interferences it will have, leading to more accurate results.
How does the concept of sensitivity relate to quality assurance?
-Sensitivity refers to the capability of the analysis to reliably and measurably respond to changes in analyte concentration. A more sensitive analysis will show a greater change in signal for a given change in analyte concentration, improving the accuracy of the results.
What is a matrix effect and how does it impact the analysis?
-A matrix effect is a change in the analyte signal caused by something in the matrix, or the environment of the analyte. It can alter the apparent concentration of the analyte, making it crucial to account for matrix effects when analyzing complex samples like river water.
How does the use of a standard addition method help in determining the initial analyte concentration?
-The standard addition method involves adding known quantities of the analyte to the unknown sample and observing how the signal changes. By comparing the signal from the initial solution to that of the final solution with the standard added, one can calculate the initial analyte concentration by extrapolating the linear curve back to the x-axis.
What is the role of an internal standard in quality assurance?
-An internal standard is a known amount of a compound that is not the analyte, added to the unknown sample. It helps account for variations in sample preparation and instrument response, ensuring that the ratio of analyte to standard remains constant and providing a more accurate measure of the analyte concentration.
How can efficient experimental design reduce the number of analyses needed to determine concentration and uncertainty?
-Efficient experimental design, such as fractional factorial design, allows for the determination of concentration and uncertainty with fewer analyses than traditional methods. By varying the ratios of the unknowns in the samples and using computer calculations, one can obtain the desired information without performing a large number of titrations.
Outlines
🔍 Introduction to Quality Assurance and Importance
This paragraph introduces the concept of quality assurance and its significance in various fields. It explains the necessity of consistent results in analysis by using an example where a sample is sent to different labs and the results vary significantly. The paragraph emphasizes the importance of precision and accuracy in measurements and defines quality assurance as the systematic monitoring and evaluation to ensure quality standards are met. It also discusses the balance between the cost of testing and the potential loss due to defective products, using the example of spaghetti sauce production. The paragraph concludes by highlighting the different levels of precision required for different products, such as a bathroom scale versus a pill, and the importance of clear objectives and specifications in achieving quality results.
📋 Sampling Techniques and Analytical Precautions
This paragraph delves into the details of sampling techniques and the precautions required for analytical procedures. It discusses the importance of collecting representative samples to ensure the accuracy of results. The paragraph uses the example of sampling from distinct regions to illustrate the need for multiple samples from each area to understand the true mean. It also touches on the concepts of selectivity (specificity) and sensitivity in analysis, explaining how these factors contribute to the quality of results. The paragraph concludes by highlighting the significance of quality assurance and quality control jobs in various industries and the importance of having trained chemists to perform these roles.
🧪 Standard Addition and Matrix Effects
This paragraph discusses the technique of standard addition, which is used to determine the initial concentration of an analyte in an unknown sample. It explains how the linear response of the analysis to the analyte concentration is crucial for this method. The concept of matrix effects is introduced, explaining how the presence of other substances in the sample can influence the analyte signal. The paragraph provides an example of measuring perchlorate in different matrices and how the results can vary significantly. It also introduces the concept of an internal standard, which is used to account for variations in sample preparation and instrument response, and explains how it helps maintain a constant ratio of analyte to standard throughout the analysis.
🧠 Efficient Experimental Design and Computational Assistance
The final paragraph focuses on the concept of efficient experimental design, which aims to obtain the desired results with the least number of trials or analyses. It uses the example of titrating three unknown acid solutions to demonstrate how fractional factorial experimental design can reduce the number of titrations needed to determine concentration and uncertainty. The paragraph explains how altering the ratios of the acids and measuring the volume of sodium hydroxide used can provide the necessary data to calculate the molarity and uncertainty of each solution. It concludes by emphasizing the role of computers in performing complex calculations, which can significantly aid in achieving efficient experimental design and reducing the burden of manual calculations.
Mindmap
Keywords
💡Quality Assurance
💡Calibration
💡Accuracy
💡Precision
💡Uncertainty
💡Sampling
💡Selectivity
💡Sensitivity
💡Matrix Effects
💡Standard Addition
💡Internal Standard
💡Efficient Experimental Design
Highlights
The importance of quality assurance in achieving consistent results across different labs is emphasized, as demonstrated by the variation in results when the same sample is analyzed by different accredited labs.
The concept of quality assurance is introduced as the systematic monitoring and evaluation to ensure standards of quality are met in projects, facilities, or services.
The analogy of making spaghetti sauce in a factory versus for friends illustrates the different levels of quality control needed based on the scale and consequences of failure.
The importance of precision and accuracy in measurements is highlighted, with the goal of quality assurance being to ensure results meet customer needs.
The difference between raw data, treated data, and results is explained, emphasizing the need for clear objectives and specifications for data and results.
The significance of understanding the acceptable rate of false positives or false negatives in different contexts, such as pharmaceuticals and food products, is discussed.
The necessity of collecting representative samples to accurately reflect the population is stressed, using the example of sampling across distinct regions.
Selectivity (specificity) and sensitivity are defined as key aspects of analytical methods, with selectivity relating to avoiding interference and sensitivity to reliably measuring changes in analyte concentration.
The role of quality assurance and quality control in various industries is highlighted, noting the demand for trained chemists and the potential for well-paying jobs in these fields.
Standard addition is introduced as a method to determine the initial analyte concentration, with the importance of a linear response to analyte concentration.
Matrix effects are explained as changes in the analyte signal caused by components of the matrix, which can impact the accuracy of measurements.
An example of how matrix effects can alter the perceived concentration of an analyte is provided, showing the impact of different sample environments on the results.
The calculation method for determining the initial analyte concentration using standard addition is detailed, including the mathematical formula.
The use of an internal standard is described as a technique to account for variations in sample preparation and instrument response, ensuring more accurate results.
Efficient experimental design is introduced as a way to obtain concentration and uncertainty values with fewer analyses than traditional methods, using fractional factorial experimental design.
The practical application of efficient experimental design is demonstrated through the example of titrating different unknown solutions of acids to determine their concentration and uncertainty.
The benefits of using computers in performing complex calculations for quality assurance and calibration methods are emphasized, highlighting the efficiency and accuracy they provide.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: