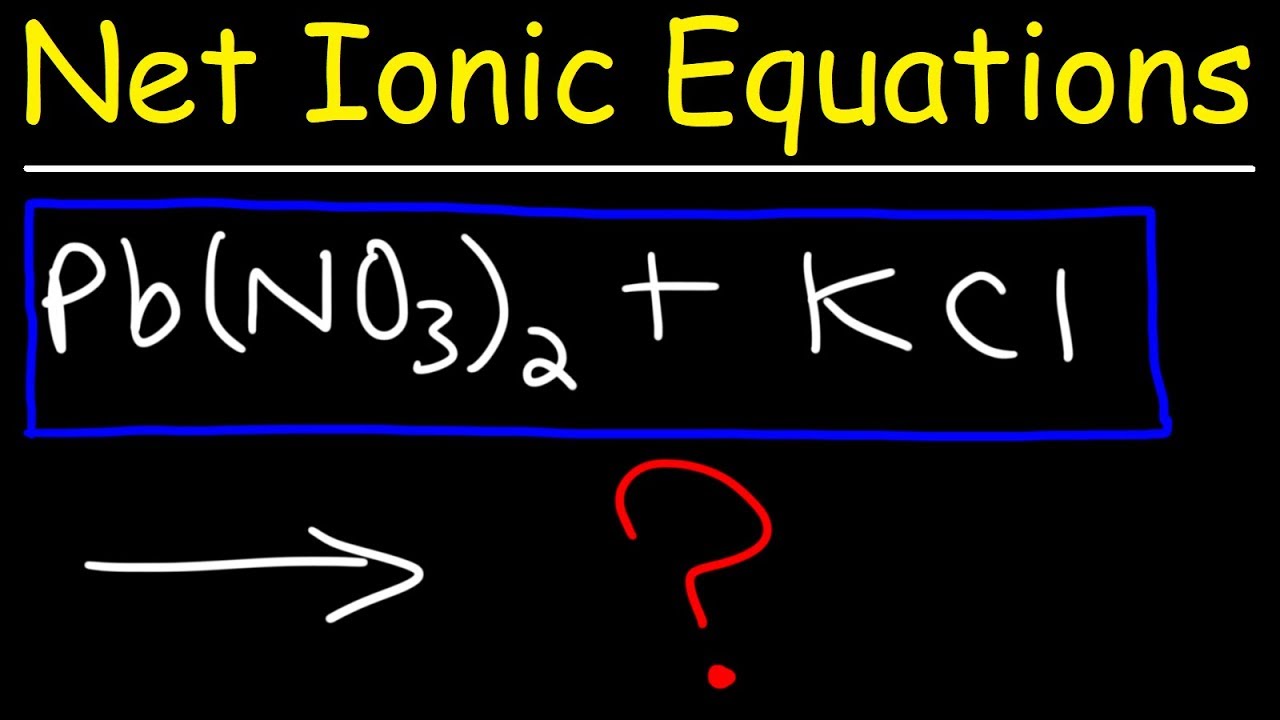
What Is Analytical Chemistry - Mr. Wizard's Quick Quiz
TLDRThe transcript describes an engaging educational activity where participants conduct a basic form of analytical chemistry through a flame test experiment. The process involves using clothespins as makeshift safety pins to dip into solutions of sodium chloride, boric acid, and strontium nitrate, and then holding them in a blowtorch flame to observe the color changes. The distinct colors emitted by each chemical—yellow for sodium chloride, green for boric acid, and red for strontium nitrate—serve as indicators for identification. This hands-on experience offers a foundational understanding of analytical chemistry techniques, such as the flame test, which is widely used by chemists to identify unknown substances.
Takeaways
- 🧪 The session involves a basic introduction to analytical chemistry, where the goal is to identify the components of unknown substances.
- 🔬 Three chemicals are used for the demonstration: sodium chloride, boric acid, and strontium nitrate, each producing a distinct flame color when heated.
- 📋 Safety is emphasized with the use of safety glasses and precautions around the blowtorch.
- 🔥 The flame test is a method used to identify substances based on the color they produce when burned.
- 💡 Sodium chloride yields a yellow flame, boric acid produces a greenish flame, and strontium nitrate results in a red flame.
- 👀 Observing the color of the flame helps in identifying the unknown substances later in the process.
- 📌 Clothespins are used as makeshift safety pins for dipping into the solutions, due to the unavailability of platinum loops.
- 🚫 Contamination is avoided by moving used probes out of the way and using clean ones for subsequent tests.
- 🤔 The process of elimination and color association helps in deducing which chemical is which based on the observed flame colors.
- 🎓 The exercise provides a hands-on experience of being an analytical chemist and can be shared with teachers and peers.
- 📈 This educational approach offers a practical understanding of a fundamental concept in chemistry, enhancing learning through visual and interactive methods.
Q & A
What is the main goal of the activity described in the transcript?
-The main goal of the activity is to demonstrate a basic principle of analytical chemistry, specifically using the flame test to identify and differentiate between various chemical substances.
Which three chemicals are mentioned in the transcript as part of the demonstration?
-The three chemicals mentioned are sodium chloride, boric acid, and strontium nitrate.
What is a flame test and how is it used in analytical chemistry?
-A flame test is a simple qualitative analytical technique used to identify the presence of certain metal ions or elements by the color they impart to a flame. It's used in analytical chemistry to differentiate between substances based on the unique color they produce when heated.
Why is the color of the flame important in the context of the transcript?
-The color of the flame is important because it helps in identifying the specific chemicals. Each chemical element or compound produces a characteristic color when it's heated, which can be used as a visual clue for analysis.
What safety measure is mentioned in the transcript?
- The safety measure mentioned is the use of safety glasses to protect the eyes from the intense light and potential sparks from the blowtorch.
Why would a platinum loop be preferred over a safety pin in a real analytical chemistry setting?
-A platinum loop would be preferred because platinum is inert and does not react with the substances being tested. It also withstands high temperatures, making it more suitable for use in flame tests compared to a safety pin, which is not designed for such conditions.
What is the significance of the yellow color in the flame test?
-The yellow color in the flame test is indicative of the presence of sodium, which is a characteristic color for sodium ions (Na+). In the context of the transcript, this color helps identify sodium chloride (table salt).
What does the green color in the flame test signify?
-The green color in the flame test is associated with the presence of either strontium or boric acid, as indicated in the transcript. This visual cue is used to differentiate between these two substances and others being tested.
How does the process described in the transcript relate to the broader field of analytical chemistry?
-The process described is a simplified demonstration of a principle used in analytical chemistry. It shows how visual observations can be used to identify unknown substances, which is a fundamental aspect of analytical chemistry that involves the development and application of methods to analyze and identify chemical compositions.
What is the purpose of the 'unknowns' in the activity?
-The 'unknowns' serve as a practical application of the knowledge gained from the flame test. By using the observed colors to identify the unknown substances, participants can apply their understanding of the relationship between flame color and chemical composition, reinforcing the concept in a hands-on manner.
How does this activity contribute to the understanding of the concept of analytical chemistry?
-This activity provides a hands-on, visual demonstration of a fundamental analytical chemistry technique. By engaging with the material in a practical way, participants can better grasp the concept of identifying and analyzing chemical substances, which is central to the field of analytical chemistry.
Outlines
🔬 Introduction to Analytical Chemistry and Flame Test
This paragraph introduces the concept of analytical chemistry, specifically focusing on the flame test experiment. It explains that the goal is to analyze chemicals to determine their composition. The experiment involves three chemicals: sodium chloride, boric acid, and strontium nitrate. The process of using a clothespin as a safety pin to dip into the solutions and then hold in a blowtorch flame to observe the color is described. The different colors exhibited by the flame (yellow for sodium chloride, greenish for boric acid, and another color for strontium nitrate) are crucial for identifying unknown samples in the future. The importance of safety measures, such as wearing safety glasses, is also highlighted. The paragraph concludes by emphasizing the educational value of this exercise in becoming a beginner analytical chemist.
Mindmap
Keywords
💡chemistry
💡analytical chemistry
💡chemist
💡sodium chloride
💡boric acid
💡strontium nitrate
💡flame test
💡safety glasses
💡blowtorch
💡color identification
💡clean probes
Highlights
Introduction to analytical chemistry and its application in identifying unknown substances.
Use of sodium chloride, boric acid, and strontium nitrate as example chemicals for analysis.
Utilization of a clothespin as a makeshift safety pin for dipping into solutions, due to lack of platinum loops.
Conducting a flame test to observe the color change in the flame, which is indicative of the chemicals' presence.
Yellow flame associated with sodium chloride.
Greenish flame indicating the presence of boric acid.
The process of elimination to identify the remaining chemical based on observed flame colors.
The educational aspect of the experiment, allowing participants to experience the role of an analytical chemist.
Safety measures, such as wearing safety glasses, before conducting the flame test.
The importance of using uncontaminated tools for accurate results in analytical chemistry.
The practical application of the flame test in identifying unknown substances in a real-world scenario.
The makeshift use of common items, like clothespins, to perform scientific experiments when specialized equipment is unavailable.
The significance of color changes in the flame as a visual indicator for the presence of specific chemicals.
The role of analytical chemistry in everyday problem-solving and its relevance beyond the classroom.
The adaptability of the experiment to be conducted with minimal equipment, making it accessible to a wider audience.
The potential for this experiment to inspire interest in chemistry and encourage further learning and exploration.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: