Ions Explained - Cations, Anions, Polyatomic Ions in Chemistry & Physics - [1-2-16]
TLDRThis lesson delves into the concept of ions in chemistry, explaining how atoms become ions through the gain or loss of electrons. It highlights the role of the periodic table in predicting an element's reactivity and its tendency to form cations or anions based on its position and the desire to achieve a stable electron configuration similar to noble gases. The lesson also touches on the strong nuclear force and why altering the nucleus is beyond the scope of chemical reactions, focusing instead on electron transfer which leads to the formation of ionic bonds.
Takeaways
- 📚 Atoms on the periodic table are neutral, having equal numbers of protons and electrons.
- 🔋 An imbalance in the number of protons and electrons results in the formation of ions.
- 💫 Ions can be positively charged (cations) or negatively charged (anions) based on the gain or loss of electrons.
- 🔌 The strong nuclear force holds protons together in the nucleus, making it extremely difficult to alter the number of protons in an atom through chemical means.
- 🌟 Noble gases have a stable electron configuration, making them unreactive, while halogens (group 17 elements) tend to gain electrons to achieve a noble gas configuration.
- 🔄 The transfer of electrons from one atom to another results in the formation of ions and subsequent ionic bonds.
- 🧪 Predicting the charge of ions can be done by understanding the position of elements on the periodic table and their tendency to gain or lose electrons.
- 🧬 The atomic number indicates the number of protons in an atom, which also equals the number of electrons in a neutral atom.
- 📈 The mass number is the sum of protons and neutrons in an atom's nucleus.
- 🔑 The net charge of an ion is determined by the difference between the number of protons and electrons.
- 🌄 The concept of ions and ionic bonding is fundamental to understanding chemical reactions and the formation of compounds.
Q & A
What is the primary topic of this lesson?
-The primary topic of this lesson is ions in chemistry, focusing on understanding what ions are, how to determine the charge on an ion, and how ions are formed.
How does an atom become an ion?
-An atom becomes an ion by either gaining or losing electrons, which results in an imbalance between the number of protons and electrons, leading to a net positive or negative charge.
What is the difference between a cation and an anion?
-A cation is an ion with a positive charge, while an anion is an ion with a negative charge. Cations form when atoms lose electrons, and anions form when atoms gain electrons.
Why can't protons be easily removed or added to an atom?
-Protons cannot be easily removed or added to an atom because they are held together in the nucleus by the strong nuclear force, which is incredibly strong and only acts at very short distances. Removing or adding a proton would require an enormous amount of energy, which is typically beyond the scope of chemical reactions and falls into the realm of nuclear physics.
What is the role of the strong nuclear force in holding the nucleus together?
-The strong nuclear force is a fundamental force of nature that is millions of times stronger than gravity. It acts within the nucleus to overcome the electrostatic repulsion between protons, holding the nucleus together despite the like charges repelling each other.
How does the periodic table help predict the formation of ions?
-The periodic table provides information about the atomic number, which tells us the number of protons in an atom's nucleus. For a neutral atom, this number also indicates the number of electrons. By knowing an element's position in the periodic table, we can predict how many electrons it will gain or lose to achieve a stable electron configuration, which in turn tells us what ion it will form.
What happens when a positively charged ion comes into contact with a negatively charged ion?
-When a positively charged ion (cation) comes into contact with a negatively charged ion (anion), they are attracted to each other due to the electrostatic force. This attraction leads to the formation of an ionic bond, resulting in a stable compound.
What is the significance of noble gases in understanding ion formation?
-Noble gases have a stable electron configuration, which makes them unreactive. They have a full outer shell of electrons, which is a lower energy state. Other elements tend to gain or lose electrons to achieve the electron configuration of the nearest noble gas, which influences the formation of ions and the types of chemical reactions they undergo.
How can you determine the charge of an ion given its symbol and the periodic table?
-The charge of an ion can be determined by its symbol, where the element's name or symbol is followed by a superscript indicating the charge. For example, a sodium ion with a positive charge is written as Na^+. By looking up the element on the periodic table, you can find its atomic number, which tells you the number of protons. For a neutral atom, this would also be the number of electrons. The charge indicates the difference between the number of protons and electrons, with a positive charge meaning fewer electrons than protons and a negative charge meaning more electrons than protons.
What is the relationship between an atom's position on the periodic table and its reactivity?
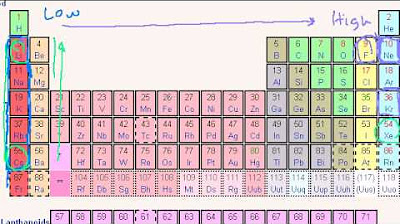
-The position of an atom on the periodic table, particularly its group, can predict its reactivity. Elements in the main groups (far left and far right of the table) tend to be more reactive because they can easily gain or lose electrons to achieve a stable electron configuration like that of the nearest noble gas. Transition metals in the center of the table are less predictable in their electron behavior and can form multiple types of ions.
Outlines
📚 Introduction to Ions in Chemistry
This paragraph introduces the concept of ions in chemistry, marking the beginning of a journey from understanding elements on the periodic table to grasping chemical reactions. It explains that atoms are neutral due to an equal number of protons and electrons, but ions are formed when there's an imbalance, either by losing or gaining electrons. The lesson aims to define what ions are, how to determine their charge, and how they are formed, which is crucial for understanding how elements bond together, such as sodium with chlorine to form sodium chloride.
🔬 Atomic Structure and Charge
The paragraph delves into the structure of atoms, emphasizing that atoms are electrically neutral because they have equal numbers of protons and electrons. It explains how the atomic number corresponds to the number of protons in the nucleus and also indicates the number of electrons in a neutral atom. The paragraph uses sodium and chlorine as examples to illustrate how atoms can become ions by gaining or losing electrons, resulting in a net charge of zero for neutral atoms and non-zero for ions.
💫 The Formation of Cations and Anions
This section explains the formation of cations and anions, which are ions with positive and negative charges, respectively. It describes how the removal or addition of electrons from an atom leads to an imbalance in charge, creating ions. The paragraph introduces the terms 'cation' for positively charged ions and 'anion' for negatively charged ions, and it explains that the charge is a result of the difference between the number of protons and electrons. The concept of nuclear forces and their role in holding the nucleus together is also briefly discussed.
🧪 Predicting Ionic Charges with the Periodic Table
The paragraph discusses how the position of an element on the periodic table can predict its tendency to gain or lose electrons to form ions. It explains that elements on the left side of the table tend to lose electrons to form cations, while elements on the right side tend to gain electrons to form anions. The goal is to achieve a stable electron configuration similar to that of noble gases. The paragraph also touches on the predictability of main group elements versus the less predictable behavior of transition metals, which can form multiple types of ions.
📝 Practice Problems: Identifying Ions and Their Charges
This paragraph presents a series of practice problems to apply the concepts learned about ions. It involves identifying the number of protons and electrons in different ions based on their charges and positions on the periodic table. The problems include determining the atomic number, the number of electrons for neutral and ionized atoms, and the net charge of the ions. The paragraph emphasizes the importance of understanding these concepts to predict the formation of chemical compounds through ionic bonding.
📋 Summary of Ions and Chemical Predictions
The final paragraph summarizes the key points of the lesson on ions. It reiterates that atoms are neutral with balanced numbers of protons and electrons, and ions are formed when there's an imbalance due to gaining or losing electrons. The periodic table's role in predicting an element's tendency to gain or lose electrons is highlighted. The lesson concludes by emphasizing that understanding ions and their charges is fundamental for predicting chemical reactions and compound formations, setting the stage for further exploration of chemical reactions in part two of the lesson.
Mindmap
Keywords
💡Ions
💡Neutral Atoms
💡Periodic Table
💡Chemical Reactions
💡Cations
💡Anions
💡Ionic Bonding
💡Electron Configuration
💡Atomic Number
💡Strong Nuclear Force
Highlights
Introduction to the concept of ions in chemistry, starting the journey from understanding elements to chemical reactions.
Explanation of atoms being neutral with equal numbers of protons and electrons, resulting in a net charge of zero.
Illustration of how an imbalance of electrons and protons leads to the formation of ions.
Clarification on the significance of ions in predicting how elements bond, using sodium and chlorine as examples.
Discussion on the atomic structure, emphasizing the solar system model's limitations and usefulness in visualizing ions.
Detailed analysis of sodium's atomic structure, including its electron cloud and nucleus composition.
Introduction of cations and anions, ions with positive and negative charges, respectively.
Explanation of why electrons can be easily added or removed from atoms, in contrast to protons due to the strong nuclear force.
Visual representation of sodium and chlorine ions, including changes in their electron count and resultant net charge.
Overview of ionic bonds formed through the attraction of oppositely charged ions, leading to compounds like sodium chloride.
Brief on the predictability of ion formation based on elements' positions on the periodic table, especially for main group elements.
Introduction to the concept of stable electron configurations and how it drives elements to gain or lose electrons.
Explanation of the reactivity of noble gases based on their electron configurations.
Insight into how transition metals' variable electron gain or loss makes their behavior less predictable.
Problem-solving examples demonstrating how to determine the number of protons, electrons, and the net charge of various ions.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: