Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (13 of 92) Time & Position Dependencies 2/3
TLDRIn this educational video, the presenter delves into the separation of variables in the Schrödinger equation, leading to two differential equations. By solving for the constant C, which represents the total energy of the particle, the wave function is expressed both as an exponential form and as a sum of sine and cosine functions. The video concludes with the promise of exploring the position-dependent solution in the upcoming segment.
Takeaways
- 📝 The script discusses the separation of variables in the Schrödinger equation, leading to two differential equations.
- 🔄 The constant of integration is introduced but remains unknown, symbolizing an undetermined factor in the equations.
- 📈 The script demonstrates the process of solving for the constant C by separating the variables and integrating both sides of the equation.
- 🧮 The natural logarithm and exponential functions are used to transform the equation into a more recognizable form.
- 🌀 The wave function is expressed in both exponential and trigonometric forms, highlighting the versatility of mathematical representations.
- 📐 The relationship between the energy of a photon (E) and the constant C is established, where C is identified as the total energy of the particle.
- 🌟 The script emphasizes the importance of the h-bar (reduced Planck constant) in the energy equation.
- 🔄 The wave function is rewritten in terms of sine and cosine functions, which are easier to interpret in the context of wave mechanics.
- 🎯 The final form of the wave function as a function of time is derived, providing a solution to the Schrödinger equation for a single particle in one dimension.
- 🚀 The script sets the stage for further exploration by indicating that the next steps will involve finding the solution to the position-dependent part of the Schrödinger equation.
Q & A
What is the main topic of the video?
-The main topic of the video is the process of solving the Schrödinger equation by separating variables for position and time, and finding the wave function for a single particle in one dimension.
What does the video start with?
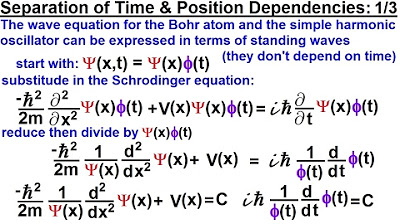
-The video starts with the separation of variables in the Schrödinger equation, leading to two differential equations, one depending only on position and the other only on time.
What is the significance of the constant that appears in the separated equations?
-The constant that appears in the separated equations is significant because it represents the energy of the particle, which is a key parameter in the wave function and will be determined later in the process.
How does the video demonstrate the solution for the time-dependent part of the wave function?
-The video demonstrates the solution for the time-dependent part by integrating the differential equation for time, leading to an expression involving the natural logarithm and eventually transforming it into an exponential form using Euler's formula.
What mathematical transformation is used to rewrite the exponential form of the wave function as a sum of sine and cosine functions?
-The mathematical transformation used is Euler's formula, which allows the exponential form to be rewritten as a sum of sine and cosine functions, with the introduction of the angular frequency term ω = 2πf.
How is the energy of a photon related to the constant C in the wave function?
-The energy of a photon is related to the constant C through the equation E = hf, where h is Planck's constant and f is the frequency. Since C is found to be equal to the total energy of the particle, it can be replaced by E in the wave function.
What does the video aim to find next?
-The video aims to find the solution to the Schrödinger equation that depends only on position, which will be the subject of the next part of the series.
Why is it necessary to solve the time-dependent part of the wave function before finding the position-dependent part?
-It is necessary to solve the time-dependent part first because the process of separating variables in the Schrödinger equation leads to two coupled equations. Solving one part allows for the determination of constants and parameters needed to solve the other part.
What is the role of the imaginary unit i in the wave function?
-The imaginary unit i is a factor in the wave function that is crucial for the phase of the wave. It appears in the sine and cosine terms and affects the oscillation behavior of the wave function over time.
How does the video use the concept of energy to find the value of the constant C?
-The video uses the concept of energy by recognizing that the constant C is related to the total energy of the particle. By equating the energy of a photon to the constant C, the video is able to determine that C is equal to the total energy E.
What is the significance of the term H-bar in the wave function?
-The term H-bar (h-bar) represents Planck's constant divided by 2π. It is a fundamental constant in quantum mechanics and appears in the wave function to ensure the correct scaling of the energy and angular frequency terms.
Outlines
📚 Solving the Wave Equation and Identifying the Constant
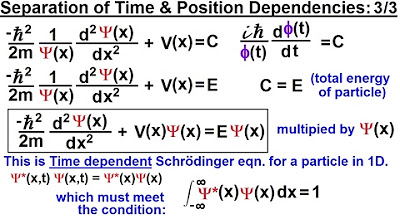
This paragraph delves into the process of solving the wave equation by separating variables and identifying the unknown constant. It explains how to derive two differential equations based on position and time, and then solve for the constant C by integrating both sides of the equation. The explanation includes the transformation of the equation into its exponential form and eventually rewriting it as a sum of sine and cosine functions. The paragraph concludes with the realization that the constant C is related to the total energy of the particle, leading to the identification of C as the energy E.
🌟 Relating Wave Function to Particle Energy and Solving the Schrödinger Equation
The second paragraph focuses on the relationship between the wave function, the energy of a photon, and the total energy of a particle. It describes how the energy E can replace the constant C in the wave function, leading to a solution for the time-dependent part of the Schrödinger equation. The paragraph also touches on the implications of this solution for understanding the behavior of a single particle in one dimension. The discussion concludes with the intention to find the solution to the position-dependent part of the Schrödinger equation in the subsequent content.
Mindmap
Keywords
💡Schrodinger Equation
💡Wave Function
💡Separation of Variables
💡Constants
💡Differential Equations
💡Integration
💡Natural Log
💡Exponential Form
💡Energy of a Photon
💡Sine and Cosine Functions
💡Quantum Mechanics
Highlights
The video begins with a recap of the previous discussion on the separation of variables in the Schrödinger equation.
Two resulting differential equations are obtained, one depending only on position and the other only on time.
The constant of integration is unknown at this stage, but the equations are set equal to it.
The process of solving for the constant C involves separating variables and integrating both sides of the equation.
The natural logarithm of the wave function with respect to time is derived from the integration process.
The antilog (exponential) of the natural logarithm gives the wave function in exponential form.
The exponential form of the wave function is then rewritten as a sum of sine and cosine functions.
The frequency (F) of the wave function is related to the constant C by C = E/H, where E is the total energy of the particle.
The energy of a photon is introduced as relevant to the discussion, with the equation E = Hν.
The constant C is identified as the total energy of the particle, leading to a clearer form of the wave function.
The wave function is expressed as a product of exponential, cosine, and sine functions, with a factor of e^(-iωt).
The solution to the Schrödinger equation for a single particle in one dimension is presented in a specific format.
The video concludes with a teaser for the next part, where the solution to the position-dependent equation will be found.
The process of solving the Schrödinger equation involves understanding the relationship between energy, frequency, and wave functions.
The video provides a step-by-step walkthrough of the mathematical manipulations required to solve the Schrödinger equation.
The use of complex numbers and Euler's formula is highlighted in the transformation of the wave function.
The video emphasizes the importance of the constant C in determining the form of the wave function.
The wave function's time-dependent part is derived in detail, providing insight into its physical interpretation.
Transcripts
Browse More Related Video
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (14 of 92) Time & Position Dependencies 3/3
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (12 of 92) Time & Position Dependencies 1/3
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (85 of 92) Transmission Coeff=? (3 of 6)
Modeling population with simple differential equation | Khan Academy
Calculus AB/BC – 7.8 Exponential Models with Differential Equations

The Theory of 2nd Order ODEs // Existence & Uniqueness, Superposition, & Linear Independence
5.0 / 5 (0 votes)
Thanks for rating: