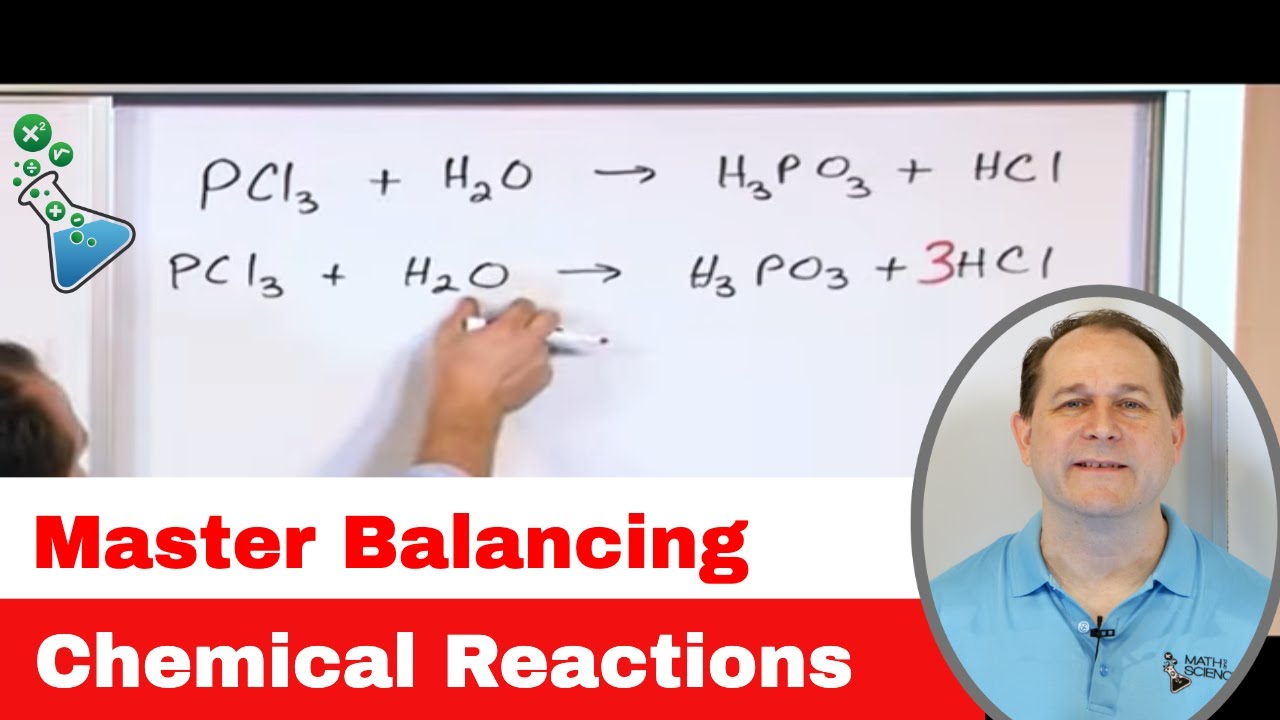
4.1 Balancing Chemical Equations| High School Chemistry
TLDRThis chemistry lesson focuses on balancing chemical reactions, a fundamental concept in high school chemistry. The instructor, Chad, explains the importance of conserving atoms in reactions and demonstrates how to balance equations for various reactions, including those involving multiple elements and phases. He also addresses common challenges, such as elements appearing in multiple places on the same side of the reaction arrow.
Takeaways
- 🧪 Balancing chemical reactions involves ensuring that the number of each type of atom on the reactant side matches the number on the product side.
- 📚 This lesson is the first in a series on chemical reactions in high school chemistry.
- 🔄 Future lessons will cover various types of chemical reactions, including oxidation-reduction reactions and single and double replacement reactions.
- 👨🏫 Chad is the instructor and the creator of the chemistry playlist, aiming to make science enjoyable and accessible.
- ⚖️ The principle behind balancing chemical reactions is the conservation of atoms—no new atoms are created or destroyed.
- 🔬 Example: Balancing sodium (Na) and chlorine (Cl2) to form sodium chloride (NaCl) involves adjusting coefficients to balance the atoms.
- 🧮 Another example: Balancing nitrogen (N2) and hydrogen (H2) to produce ammonia (NH3) by adjusting coefficients to balance the atoms on both sides.
- ❗ A more complex example involves balancing a reaction where an element appears in multiple compounds on the same side, making the process more challenging.
- ✍️ Strategy: Start with elements that appear in only one compound on each side of the equation and leave elements that appear in multiple compounds for last.
- 🌟 Support: Viewers are encouraged to like, share, and check out premium courses for more practice and study guides.
Q & A
What is the main topic of this lesson in the high school chemistry playlist?
-The main topic of this lesson is balancing chemical reactions.
What are the two important types of chemical reactions that will be focused on after this lesson?
-The two important types of chemical reactions that will be focused on are oxidation-reduction reactions and double replacement reactions.
What is the principle behind balancing chemical reactions?
-The principle behind balancing chemical reactions is the conservation of atoms, ensuring that all atoms present on the reactant side are still present on the product side, just in different combinations.
How does the instructor suggest starting to balance a chemical reaction?
-The instructor suggests that you can start balancing a chemical reaction with any element you want, but cautions that there are some reactions where the starting element matters and certain places where you should not start.
What is the reaction between solid sodium metal and chlorine gas?
-The reaction between solid sodium metal and chlorine gas produces solid sodium chloride, which is table salt.
Why is the reaction between sodium metal and chlorine gas described as highly exothermic?
-The reaction is described as highly exothermic because it releases a lot of heat and can even produce flames during the process.
What is the process of balancing the reaction between N2 gas and H2 gas to produce ammonia?
-To balance the reaction, you start by ensuring the nitrogen atoms are balanced by placing a coefficient of 2 in front of NH3. Then, you balance the hydrogen atoms by adding a coefficient of 3 in front of H2, resulting in 2 N2 + 3 H2 producing 2 NH3.
What is the challenge when balancing a chemical reaction with an element that appears in more than one place on the same side of the reaction arrow?
-The challenge is that it becomes difficult to balance the reaction, as you may end up going back and forth without achieving a balanced equation. It's recommended to make such elements the last to balance.
Why might the instructor suggest doubling all coefficients in a balanced chemical equation?
-The instructor might suggest doubling all coefficients to avoid fractions and ensure that all coefficients are whole numbers, which is a common requirement in chemistry classes.
What is the final balanced equation for the reaction involving C2H6, O2, CO2, and H2O?
-The final balanced equation, avoiding fractions, is 2 C2H6 + 7 O2 producing 4 CO2 + 6 H2O.
What is the instructor's advice for students who find the lesson helpful?
-The instructor advises students who find the lesson helpful to give a like, share it, and check out the premium course on chatsprep.com for practice and study guides.
Outlines
🧪 Balancing Chemical Reactions in High School Chemistry
This paragraph introduces the concept of balancing chemical reactions, a fundamental aspect of high school chemistry. The speaker, Chad, welcomes viewers to his channel, which aims to make science enjoyable. He explains that balancing chemical reactions involves ensuring that the number of atoms of each element is conserved from the reactant side to the product side. The process is illustrated with the example of reacting solid sodium with chlorine gas to form sodium chloride (table salt). Chad emphasizes the importance of this lesson, noting that it is a key part of students' chemistry education. He also provides a brief overview of the upcoming lessons in the playlist, which will cover various types of chemical reactions, including oxidation-reduction and single and double replacement reactions.
🔍 Strategies for Balancing Complex Chemical Reactions
This paragraph delves into the more complex aspects of balancing chemical reactions, particularly when elements appear in multiple places on the same side of the reaction. Chad advises starting with elements that appear only once on each side of the reaction arrow, such as carbon and hydrogen in the given example. He demonstrates how to balance a reaction involving ethene (C2H4), carbon dioxide (CO2), and water (H2O), highlighting the challenges posed by oxygen, which appears in multiple places on the product side. Chad explains that starting with oxygen would complicate the balancing process, so it should be addressed last. He also discusses the option of doubling coefficients to avoid fractions, which is a common practice in chemistry education. The paragraph concludes with a reminder to avoid fractions in coefficients, if possible, and encourages viewers to support the channel by liking and sharing the content.
Mindmap
Keywords
💡Balancing Chemical Reactions
💡Conservation of Mass
💡Reactants
💡Products
💡Coefficients
💡Phase
💡Oxidation-Reduction Reactions
💡Single Replacement Reactions
💡Double Replacement Reactions
💡Exothermic Reaction
💡Ammonia
Highlights
Balancing chemical reactions is a fundamental concept in high school chemistry.
The lesson introduces the concept of balancing chemical reactions and its importance in understanding chemical changes.
All elements on the reactant side must be balanced with corresponding ones on the product side, ensuring atom conservation.
The example of mixing solid sodium metal with chlorine gas to form sodium chloride illustrates the balancing process.
The reaction between sodium and chlorine is highly exothermic, releasing heat and flames.
Balancing a chemical reaction involves adjusting coefficients to ensure equal numbers of atoms on both sides.
The example of nitrogen and hydrogen gases forming ammonia gas demonstrates balancing with diatomic elements.
Cooling the reaction below -33 degrees Celsius can result in liquid ammonia, highlighting the importance of temperature in reactions.
Balancing reactions with multiple chemical species requires careful consideration of where to start the balancing process.
Elements appearing in more than one place on the same side of the reaction should be balanced last to avoid complexity.
The example of carbon and hydrogen in ethane and oxygen in carbon dioxide and water illustrates the complexity of balancing.
Fractions in coefficients can be avoided by doubling all coefficients to achieve whole numbers.
The final balanced chemical reaction must have whole number coefficients for all elements.
The instructor emphasizes the importance of practice and understanding the principles behind balancing chemical reactions.
The channel offers a high school chemistry playlist with lessons released throughout the 2020-21 school year.
Subscribers are encouraged to hit the bell notifications to stay updated with new lessons.
The instructor provides additional resources and study guides through a premium course on chatsprep.com.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: