Hess's law example | Thermodynamics | Chemistry | Khan Academy
TLDRThe video script discusses using Hess's Law to calculate the enthalpy change for the formation of methane from solid carbon (graphite) and hydrogen gas. It explains how to reverse and combine combustion reactions of carbon, hydrogen, and methane to derive the desired reaction, ultimately determining the enthalpy change as -74.8 kJ/mol.
Takeaways
- 🔍 The problem involves calculating the enthalpy change for the formation of methane (CH4) from solid carbon (graphite) and hydrogen gas.
- 🚫 Direct measurement of the enthalpy change for this reaction in the lab is not feasible due to its slow nature.
- 🔥 Hess's Law is used to determine the enthalpy change for the formation of methane by combining the enthalpy changes of other known reactions.
- 🌐 Hess's Law states that the enthalpy change of a reaction is the sum of the enthalpy changes of the reactions it is composed of.
- 🔄 The script suggests reversing the combustion reaction of methane to start with the end product (methane) and then adding other reactions to construct the desired reaction.
- ⚖️ The combustion reactions for carbon and hydrogen are used, and their enthalpy changes are adjusted based on the stoichiometry of the reactions.
- 🔄 The combustion of carbon (graphite) produces carbon dioxide, and the combustion of hydrogen gas produces water, which are necessary intermediates in the formation of methane.
- 🔢 The script demonstrates the process of multiplying the hydrogen combustion reaction by 2 to obtain the required two molecules of water.
- 🔄 The script shows how the reactants and products of the combined reactions cancel out, leaving only the desired reactants (graphite and hydrogen gas) and the product (methane).
- 📊 The final enthalpy change for the formation of methane is calculated by summing the adjusted enthalpy changes of the individual reactions, resulting in a negative value indicating an exothermic process.
Q & A
What is the main problem being addressed in the script?
-The main problem is to calculate the enthalpy change for the formation of methane (CH4) from solid carbon (graphite) and hydrogen gas, using Hess's Law.
Why can't the enthalpy change for the formation of methane be measured directly in the laboratory?
-The enthalpy change cannot be measured directly because the reaction is very slow, making it difficult to measure the temperature change or any meaningful data in the lab.
What method is suggested to find the enthalpy change for the formation of methane?
-The suggested method is to use Hess's Law, which involves using the enthalpy changes of related combustion reactions to find the enthalpy change for the formation of methane.
What are the given reactions and their enthalpy changes used in the script?
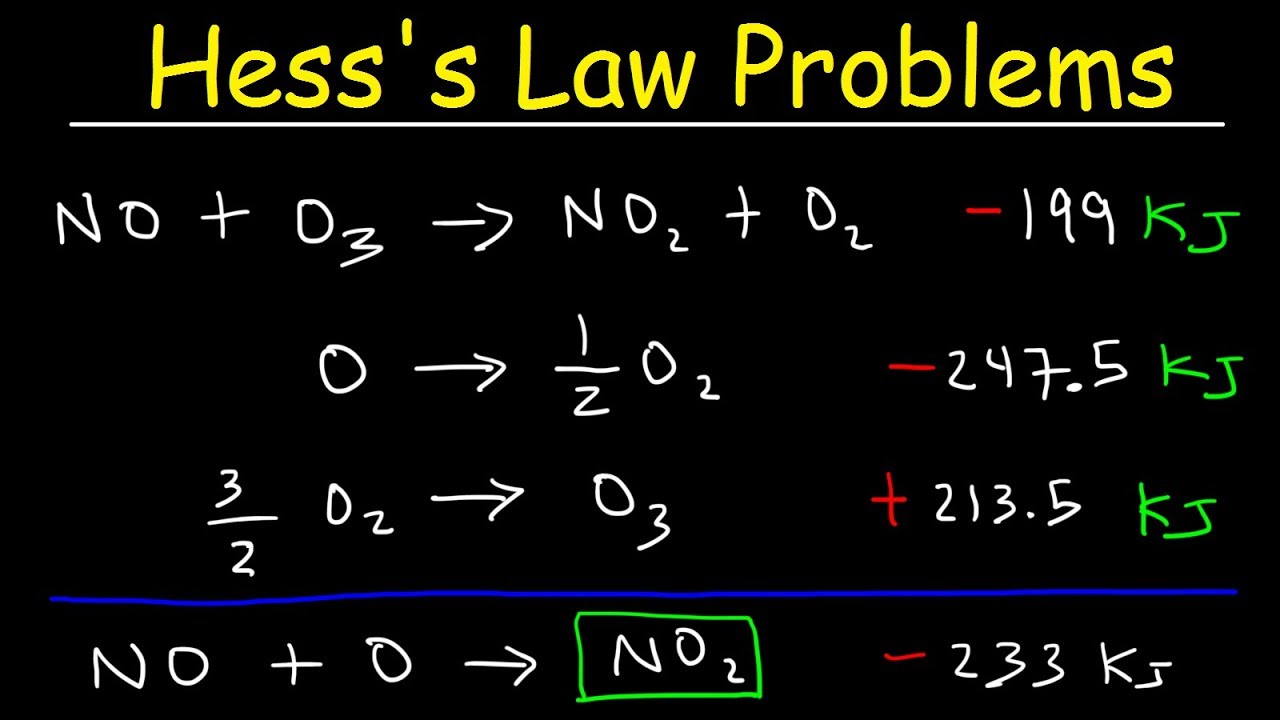
-The given reactions and their enthalpy changes are: 1. Combustion of carbon: C (graphite) + O2 -> CO2, ΔH = -393.5 kJ/mol 2. Combustion of hydrogen: H2 + 1/2 O2 -> H2O, ΔH = -285.8 kJ/mol 3. Combustion of methane: CH4 + 2 O2 -> CO2 + 2 H2O, ΔH = -890.3 kJ/mol
How is Hess's Law applied in this problem?
-Hess's Law is applied by reversing the combustion reaction of methane and combining it with the combustion reactions of carbon and hydrogen in such a way that the intermediate products cancel out, leaving the formation reaction of methane.
Why do we reverse the combustion reaction of methane?
-We reverse the combustion reaction of methane because in the original problem, methane is a product, not a reactant. Reversing the reaction gives us the formation of methane as a product.
How do we handle the stoichiometry in this Hess's Law problem?
-We ensure that the stoichiometry of the intermediate products matches the target reaction. Specifically, we multiply the combustion reaction of hydrogen by 2 to get the correct amount of water (2 H2O) needed.
What is the total enthalpy change for the formation of methane, according to the script?
-The total enthalpy change for the formation of methane is -74.8 kJ/mol.
What is the significance of a negative enthalpy change in this context?
-A negative enthalpy change indicates that the reaction is exothermic, meaning it releases energy.
What is the final form of the reaction for the formation of methane after applying Hess's Law?
-The final form of the reaction is: C (graphite) + 2 H2 (gas) -> CH4 (gas).
Outlines
🔥 Calculating Enthalpy Change for Methane Formation
The script discusses a problem from the Kotz, Treichel, Townsend Chemistry textbook, focusing on calculating the enthalpy change for the formation of methane from solid carbon (graphite) and hydrogen gas. The enthalpy change for this reaction is not directly measurable due to its slow nature. Instead, the problem suggests using Hess's Law, which states that the enthalpy change of a reaction is the sum of the enthalpy changes of the reactions it is composed of. The script proposes starting with the combustion of methane, reversing the reaction to represent methane as a product, and then using the enthalpy changes of the combustion of carbon and hydrogen to construct the desired reaction. The goal is to determine the enthalpy change for the formation of methane from its elements.
🔍 Constructing the Reaction Using Combustion Data
This paragraph delves deeper into the process of using Hess's Law to calculate the enthalpy change for methane formation. It explains how to reverse the combustion reaction of methane and multiply the combustion reaction of hydrogen by two to match the required stoichiometry for water in the formation of methane. The script details how to combine these reactions, ensuring that reactants and products cancel out appropriately, leaving only the desired reactants (graphite and hydrogen gas) and the product (methane). The enthalpy changes for the individual reactions are then used to calculate the overall enthalpy change for the formation of methane.
📚 Applying Hess's Law to Determine Enthalpy Change
The final paragraph concludes the process by applying Hess's Law to sum the enthalpy changes of the individual reactions calculated in the previous paragraphs. It emphasizes the importance of reversing the combustion reaction of methane and adjusting the reaction of hydrogen combustion to fit the stoichiometry needed for methane formation. The enthalpy changes for the combustion of carbon (-393.5 kJ/mol) and hydrogen (-571.6 kJ/mol) are combined with the reversed methane combustion reaction (+890.3 kJ/mol) to find the net enthalpy change for the formation of methane. The result is a negative enthalpy change of -74.8 kJ/mol, indicating that the formation of methane is exothermic, though not as significantly as the combustion reactions.
Mindmap
Keywords
💡Enthalpy Change
💡Methane (CH4)
💡Graphite
💡Hydrogen Gas
💡Combustion Reaction
💡Hess's Law
💡Endothermic Reaction
💡Exothermic Reaction
💡Carbon Dioxide (CO2)
💡Water (H2O)
💡Kilojoules
Highlights
Introduction of the problem from Chapter 5 of the Kotz, Treichel, Townsend Chemistry and Chemical Reactivity textbook.
Explanation of the goal: to find the enthalpy change for the formation of methane (CH4) from solid carbon as graphite and hydrogen gas.
Clarification that the enthalpy change for this reaction cannot be measured in the laboratory due to the slow reaction rate.
Introduction of Hess's Law as a method to calculate the enthalpy change indirectly.
Step-by-step approach to constructing the desired reaction using given combustion reactions.
Reversal of the combustion of methane reaction to have methane as a product.
Explanation that reversing the reaction changes the sign of the enthalpy change to +890.3 kJ/mol.
Combustion of graphite to form carbon dioxide with an enthalpy change of -393.5 kJ/mol.
Combustion of hydrogen gas to form water with an enthalpy change of -285.8 kJ/mol.
Multiplication of the hydrogen combustion reaction by 2 to match the number of water molecules needed.
Calculation of the enthalpy change for the multiplied hydrogen combustion reaction: -571.6 kJ/mol.
Verification that the sum of the reactions cancels out all intermediate products, leaving the desired reactants and products.
Summation of the enthalpy changes from the individual reactions to find the total enthalpy change for the formation of methane.
Final calculation showing the enthalpy change for the formation of methane as -74.8 kJ/mol, indicating an exothermic reaction.
Conclusion that the enthalpy change calculation using Hess's Law confirms the exothermic nature of the methane formation reaction.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: