Introduction to Oxidation States
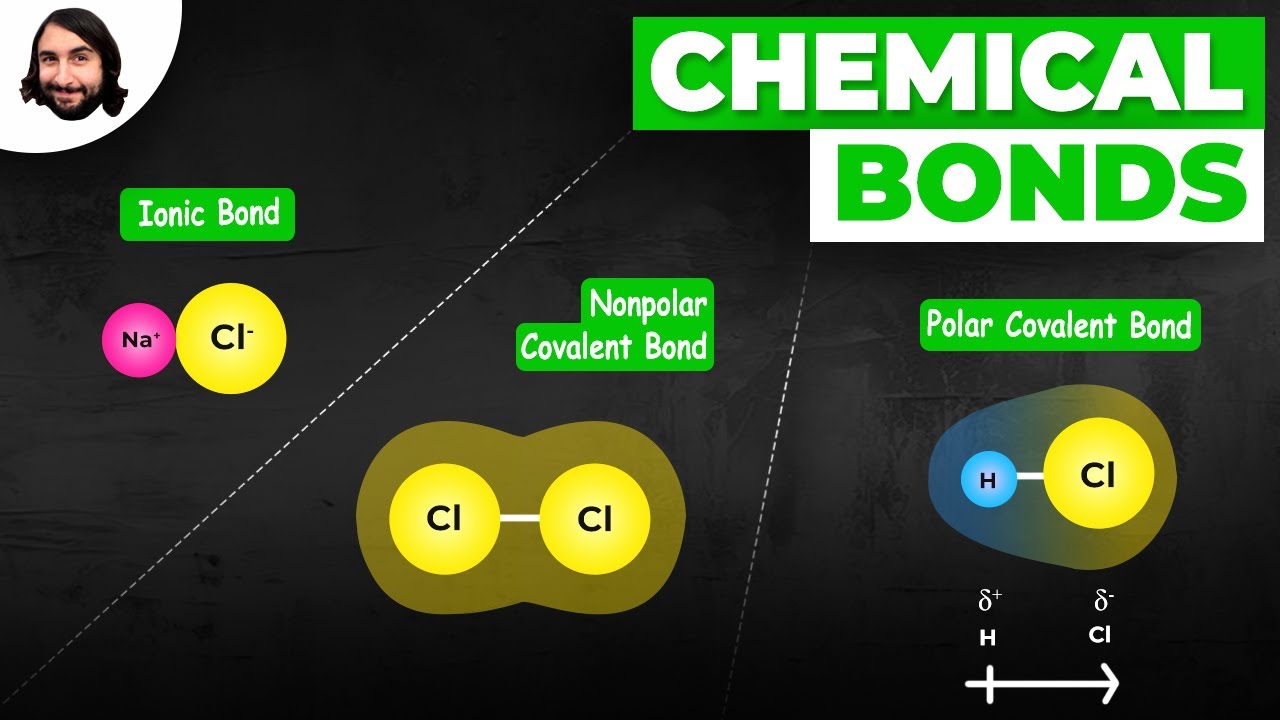
TLDRThis chemistry lesson delves into the concepts of ionic and covalent bonding, using sodium chloride and water molecules as examples. It explains how sodium, an alkali metal, loses an electron to form a +1 charge, while chlorine, a halogen, gains an electron to achieve a full outer shell with a -1 charge, forming an ionic bond. The script also introduces oxidation states as a hypothetical tool to understand electron distribution in covalent bonds, particularly highlighting how electronegative atoms like oxygen and fluorine pull electrons towards them, creating partial charges and leading to phenomena like hydrogen bonding. The importance of understanding oxidation and reduction in chemical reactions is emphasized, with a clear explanation of how oxidation states can be used to predict the behavior of elements in compounds.
Takeaways
- 🌟 Sodium (Na) is an alkali metal in Group 1 with one valence electron, which it tends to lose to achieve stability.
- 🌟 Chlorine (Cl) is a halogen that needs one electron to complete its octet, making it electronegative and likely to gain an electron.
- 🌟 The interaction between sodium and chlorine results in an ionic bond, where sodium loses an electron and chlorine gains one, forming Na+ and Cl- ions.
- 🌟 In ionic bonds, there is a complete transfer of electrons from one atom to another, leading to charged ions that attract each other due to Coulomb's force.
- 🌟 Oxygen is highly electronegative and tends to pull electrons towards itself in covalent bonds, creating partial charges and leading to phenomena like hydrogen bonding.
- 🌟 Oxidation states are hypothetical charges assigned to atoms in compounds, representing what their charges would be if the bonds were ionic.
- 🌟 Oxidation refers to the loss of electrons, leading to a positive charge, while reduction refers to the gain of electrons, leading to a negative charge.
- 🌟 The oxidation state of an atom in a compound can help predict its behavior in chemical reactions, even in covalent bonds where electrons are shared.
- 🌟 The sum of oxidation states in a neutral molecule equals zero, and in a charged molecule, the sum equals the overall charge of the molecule.
- 🌟 Elements in the periodic table have typical oxidation states based on their position and electronegativity, which can be used to predict the oxidation states in various compounds.
Q & A
What type of bond is formed between sodium and chlorine in sodium chloride?
-An ionic bond is formed between sodium and chlorine in sodium chloride. Sodium, being an alkali metal, loses its one valence electron, and chlorine, a halogen, gains that electron to complete its octet, resulting in sodium having a +1 charge and chlorine having a -1 charge.
Why does sodium want to lose its valence electron?
-Sodium, being in Group 1 of the periodic table, has one valence electron and tends to lose it to achieve a stable electron configuration similar to the noble gas preceding it, in this case, neon.
How does the electronegativity of oxygen affect its bonding with hydrogen in water (H2O)?
-Oxygen is more electronegative than hydrogen, which means it has a greater tendency to attract electrons. In a water molecule, oxygen forms a covalent bond with hydrogen but pulls the shared electrons closer to itself, creating a partial negative charge on oxygen and partial positive charges on the hydrogens.
What is the concept of oxidation states and how do they differ from actual charges in covalent bonds?
-Oxidation states are hypothetical charges assigned to atoms in a compound, assuming that the more electronegative atom has gained electrons and the less electronegative atom has lost them. This differs from actual charges in covalent bonds, where electrons are shared rather than transferred.
What is the oxidation state of hydrogen in a compound with oxygen, like in water?
-In a compound with oxygen, such as water (H2O), the oxidation state of hydrogen is +1. This is because oxygen is more electronegative and attracts the shared electrons, hypothetically 'removing' them from hydrogen, which would then be considered to have lost an electron.
How does the oxidation state of an element relate to its position on the periodic table?
-The oxidation state of an element is related to its position on the periodic table because elements' tendencies to gain or lose electrons are influenced by their electronegativity, which generally increases from left to right across a period and decreases down a group.
What is the oxidation state of oxygen in most compounds?
-In most compounds, the oxidation state of oxygen is -2, reflecting its tendency to gain two electrons to achieve a stable electron configuration similar to that of the noble gas neon.
What is the oxidation state of hydrogen when it bonds with metals like lithium?
-When hydrogen bonds with metals like lithium, its oxidation state is -1. This is because hydrogen is more electronegative than lithium and will gain the electron from lithium, which loses its electron to achieve stability.
What is the sum of the oxidation states in a neutral compound?
-In a neutral compound, the sum of the oxidation states of all the atoms equals zero, reflecting the overall electrical neutrality of the compound.
Can you provide an example where oxygen does not have its typical oxidation state of -2?
-While the script does not provide a specific example, there are cases where oxygen does not exhibit its typical -2 oxidation state, such as in peroxides (e.g., H2O2) where oxygen has an oxidation state of -1.
What is the mnemonic 'GER' used for in the context of oxidation and reduction?
-The mnemonic 'GER' stands for 'Losing Electrons is Oxidation, Gaining Electrons is Reduction', helping to remember that oxidation involves the loss of electrons (resulting in a positive charge) and reduction involves the gain of electrons (resulting in a negative charge).
Outlines
🧪 Ionic and Covalent Bonds in Chemistry
This paragraph explains the formation of ionic and covalent bonds using sodium chloride (NaCl) as an example. Sodium, an alkali metal from Group 1, tends to lose its single valence electron, resulting in a +1 charge. Chlorine, a halogen, needs one electron to complete its valence shell and thus accepts the electron from sodium, gaining a -1 charge. This electron transfer creates an ionic bond due to the electrostatic attraction between the oppositely charged ions. The concept is contrasted with covalent bonding in water (H2O), where oxygen, being more electronegative, pulls the shared electrons closer, creating a partial negative charge on oxygen and partial positive charges on hydrogen. This leads to hydrogen bonding. The paragraph introduces oxidation states as a hypothetical assignment of charges to atoms in covalent bonds, illustrating with water and hydrogen fluoride (HF) molecules.
🔍 Understanding Oxidation and Reduction
The second paragraph delves into the concepts of oxidation and reduction. Oxidation is described as the hypothetical loss of electrons, symbolized by a positive charge, which does not necessarily involve oxygen. For instance, in hydrogen fluoride (HF), fluorine, being more electronegative, takes the electron from hydrogen, 'oxidizing' it. Reduction, conversely, is the gain of electrons, resulting in a negative charge, which is exemplified by fluorine gaining an electron from hydrogen. The paragraph clarifies that oxidation and reduction are not solely dependent on the presence of oxygen and introduces the mnemonic 'Leo the lion says GER' to remember that Losing Electrons is Oxidation and Gaining Electrons is Reduction.
📊 Oxidation States and Their Calculation
This paragraph discusses the assignment of oxidation states to atoms in a compound, which helps in understanding the hypothetical ionic character of covalent bonds. It explains that oxidation states are based on the electronegativity of elements, with more electronegative atoms assigned a negative oxidation state and less electronegative atoms a positive one. The paragraph uses examples from the periodic table, such as alkali metals which typically have a +1 oxidation state, and halogens which usually have a -1 state. It also touches on hydrogen's variable oxidation state, which can be +1 or -1 depending on whether it is bonded to a more or less electronegative element, respectively. The importance of the sum of oxidation states in a neutral compound equaling zero is highlighted, with examples provided to illustrate this principle.
🌐 Advanced Oxidation States and Periodic Trends
The final paragraph expands on the concept of oxidation states, discussing the typical states for various groups in the periodic table. It emphasizes that alkaline earth metals tend to have a +2 oxidation state due to their tendency to lose two electrons. The paragraph also revisits hydrogen, noting its unique position with either a +1 or -1 oxidation state based on its bonding partner. Oxygen's common -2 oxidation state is reiterated, with an exception mentioned for a special case that will be covered in a subsequent video. The halogens' preference for a -1 oxidation state is also noted. The paragraph concludes by stressing the ability to deduce the oxidation states of most elements in a compound, given the known states of hydrogen, oxygen, and halogens, and sets the stage for more complex examples in future videos.
Mindmap
Keywords
💡Sodium chloride
💡Valence electron
💡Halogen
💡Ionic bond
💡Electronegativity
💡Covalent bond
💡Oxidation state
💡Oxidation
💡Reduction
💡Alkali metals
💡Neutral molecule
Highlights
Sodium chloride forms an ionic bond due to sodium's desire to lose its one valence electron and chlorine's need for one more electron to complete its octet.
Sodium ends up with a +1 charge after losing an electron, and chlorine with a -1 charge after gaining an electron, illustrating the formation of an ionic bond.
In contrast to ionic bonds, covalent bonds involve sharing of electrons, as seen in the case of H2O where oxygen pulls electrons towards itself due to its higher electronegativity.
The concept of oxidation states is introduced as a hypothetical scenario to understand the distribution of electrons in covalent bonds.
Oxidation states help in understanding reactions by assigning hypothetical charges to atoms based on their electronegativity.
Oxidation is defined as the hypothetical loss of electrons, leading to a positive charge, while reduction is the gain of electrons, resulting in a negative charge.
The mnemonic 'Leo the lion says GER' is used to remember that Losing Electrons is Oxidation and Gaining Electrons is Reduction.
Alkali metals typically have an oxidation state of +1 as they tend to lose their outermost electron to achieve stability.
Hydrogen's oxidation state can vary between +1 and -1 depending on whether it is bonding with more or less electronegative elements.
In the case of lithium hydride, lithium is oxidized and hydrogen is reduced, resulting in oxidation states of +1 for lithium and -1 for hydrogen.
For neutral molecules, the sum of oxidation states equals zero, reflecting the balance of charges.
In non-neutral compounds, the sum of oxidation states equals the overall charge of the compound.
Oxygen typically has an oxidation state of -2, reflecting its tendency to gain two electrons in most compounds.
Halogens generally have an oxidation state of -1, as they tend to gain one electron to achieve a stable configuration.
The oxidation state of an element in a compound can be predicted based on its position in the periodic table and its electronegativity.
An exception to oxygen's usual -2 oxidation state is demonstrated, indicating that specific conditions can alter the typical oxidation state.
The importance of understanding oxidation states for predicting the behavior of elements in chemical reactions is emphasized.
Transcripts
Browse More Related Video

Oxidation and reduction | Redox reactions and electrochemistry | Chemistry | Khan Academy

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy

Introduction to Ionic Bonding and Covalent Bonding

Polar and Nonpolar Covalent Bonds

ATI TEAS 7 I Chemical Bonds I Chemistry I
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
5.0 / 5 (0 votes)
Thanks for rating: