Suspensions, colloids and solutions | Chemistry | Khan Academy
TLDRThis script delves into the chemistry of mixtures, focusing on homogeneous mixtures exemplified by homogenized milk. It explains the difference between suspensions, colloids, and solutions based on particle size, using relatable examples like paint and chocolate milk. The video also explores the concepts of solute and solvent, and introduces various methods of measuring concentration, such as mole fraction, molarity, and molality, highlighting the importance of understanding these distinctions in chemistry.
Takeaways
- 🧪 Mixing is a fundamental process in chemistry, often involving the creation of various types of mixtures.
- 🥛 Homogenized milk is an example of a homogeneous mixture, where fat is evenly distributed throughout the milk to prevent separation.
- 🔍 Homogeneous mixtures are uniform and consistent, without significant variation within the mixture itself.
- 🌌 Suspensions are mixtures where particles larger than 500 nanometers are mixed but will eventually settle or float due to gravity.
- 🎨 Examples of suspensions include paint that needs to be shaken before use and chocolate milk which can separate over time.
- 🌫 Colloids are homogeneous mixtures with particles ranging from 2 to 500 nanometers, which remain suspended due to intermolecular forces.
- 🍮 Common examples of colloids include Jell-O and fog, where the particles do not settle out over time.
- 💧 Solutions are homogeneous mixtures with particles smaller than 2 nanometers, such as when sodium chloride is dissolved in water.
- 🔬 In solutions, solute particles like sodium and chloride ions are attracted to the oppositely charged ends of water molecules, facilitating dissolution.
- ⚖️ Mole fraction is a method to measure concentration, calculated as the number of moles of solute divided by the total number of moles in the solution.
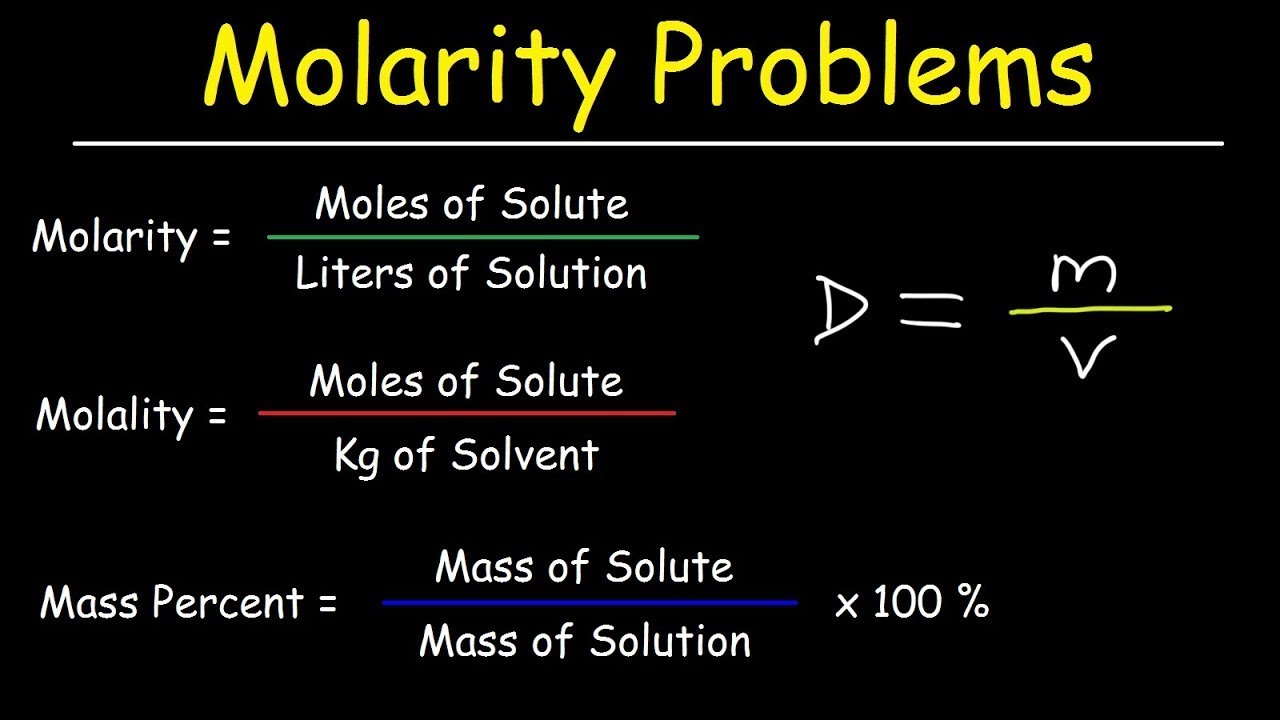
- 📏 Molarity and molality are two different measures of concentration; molarity is moles of solute per liter of solution, while molality is moles of solute per kilogram of solvent.
Q & A
What is a homogeneous mixture?
-A homogeneous mixture is a type of mixture that is uniform or consistent throughout, with no significant variation in its composition.
Why is homogenized milk different from regular milk?
-Homogenized milk is processed to disperse the fat evenly throughout the milk, preventing a layer of fat from separating at the top and making it creamy and consistent in texture.
What is the significance of 500 nanometers in the context of mixtures?
-In mixtures, if the particles are larger than 500 nanometers, they form a suspension, which will eventually settle or separate out unless agitated.
What is a suspension and how is it different from a colloid?
-A suspension is a mixture where particles larger than 500 nanometers are dispersed but will eventually settle or float due to gravity. A colloid, on the other hand, has particles between 2 and 500 nanometers that remain suspended due to intermolecular forces.
What are some examples of colloids mentioned in the script?
-Examples of colloids include Jell-O, fog, and smoke. These mixtures have particles that are small enough to stay suspended without settling.
What is the difference between a solution and a colloid in terms of particle size?
-A solution has particles smaller than 2 nanometers, which are completely dissolved and do not separate out. A colloid has particles between 2 and 500 nanometers, which are suspended but not dissolved.
How does the script explain the dissolution of sodium chloride in water?
-The script explains that when sodium chloride is dissolved in water, the positively charged sodium ions are attracted to the partially negative oxygen ends of water molecules, and the negatively charged chloride ions are attracted to the partially positive hydrogen ends, allowing the salt to dissolve.
What is the role of polarity in the dissolution process of a solute in water?
-Polarity plays a crucial role in the dissolution process as it allows charged particles of the solute to interact with the polar water molecules, leading to the separation of ions and their dispersion within the water.
What are the units of measurement for molality and molarity?
-Molality is measured in moles of solute per kilogram of solvent (moles/kg), while molarity is measured in moles of solute per liter of solution (moles/L).
Why might molality be considered a more stable measure of concentration than molarity?
-Molality is considered more stable because it is based on the mass of the solvent, which remains constant regardless of changes in temperature or pressure, unlike the volume of the solution which can change and affect molarity.
What is the mole fraction and how is it used in the script?
-The mole fraction is the ratio of the number of moles of solute to the total number of moles in the solution. In the script, it is used to calculate partial pressures in a mixture by multiplying the mole fraction by the total pressure.
Outlines
🥛 Homogenized Milk and Homogeneous Mixtures
The first paragraph introduces the concept of homogeneous mixtures, which are uniform and consistent throughout. Homogenized milk is used as an example to explain this concept. Regular milk, freshly milked from a cow or goat, quickly separates into a layer of fat and a more liquid portion. Homogenization ensures that the fat is evenly dispersed throughout the milk, resulting in a creamy texture without a layer of fat on top. This process is contrasted with suspensions, where particles larger than 500 nanometers settle or float over time, requiring agitation to maintain a uniform mixture. Examples of suspensions include mixed paint and chocolate milk, which separate if left undisturbed. The paragraph also distinguishes between suspensions and colloids, which involve particles between 2 to 500 nanometers, and solutions, which involve particles smaller than 2 nanometers.
🌫️ Colloids, Aerosols, and Solutions
The second paragraph delves deeper into the types of homogeneous mixtures, focusing on colloids and solutions. Colloids are mixtures where particles are small enough to remain suspended, such as gelatin in Jell-O, fog, and smoke. Aerosols are a type of colloid where the dispersed particles are either liquid (fog) or solid (smoke) in a gaseous medium. Solutions are defined as mixtures where the solute particles are less than 2 nanometers in size, making them completely dissolved in the solvent. The paragraph also discusses the importance of distinguishing between colloids and solutions in everyday life and in chemistry, emphasizing that most chemical reactions involve solutions. The example of sodium chloride dissolving in water is used to illustrate how ions interact with water molecules, leading to dissolution.
🧪 Measuring Concentration in Solutions
The third paragraph discusses various methods of measuring the concentration of solutions, highlighting the differences between mole fraction, molarity, and molality. Mole fraction is calculated by dividing the number of moles of solute by the total number of moles in the solution. Molarity is defined as the moles of solute per liter of solution, which can vary with changes in pressure and temperature. Molality, on the other hand, is the moles of solute per kilogram of solvent, making it a more constant measure of concentration regardless of external conditions. The speaker suggests that molality is a more reliable measure due to its invariance to changes in volume and pressure. The paragraph concludes with a challenge for viewers to come up with mnemonics to remember the difference between molarity and molality.
Mindmap
Keywords
💡Homogenized
💡Homogeneous Mixture
💡Suspension
💡Colloid
💡Aerosol
💡Solution
💡Solute
💡Solvent
💡Mole Fraction
💡Molarity
💡Molality
Highlights
Introduction to the concept of homogeneous mixtures and their uniformity throughout.
Explanation of homogenized milk as a common example of a homogeneous mixture.
Difference between milk fat and non-milk fat and how they separate in non-homogenized milk.
Process of homogenization in milk to ensure even fat distribution.
Characteristics of suspensions with particles larger than 500 nanometers and their tendency to separate over time.
Examples of suspensions, including mixed paint and chocolate milk, and the need to shake them to maintain homogeneity.
Definition and characteristics of colloids, with particle sizes between 2 and 500 nanometers that remain suspended.
Examples of colloids such as Jell-O, fog, and smoke, and their stability in mixtures.
Introduction to solutions with particle sizes below 2 nanometers, which are completely dissolved and homogeneous.
Importance of solutions in chemistry and the concept of aqueous solutions.
Explanation of solute and solvent in the context of solutions and their interaction.
Role of polarity in water allowing the dissolution of ionic compounds.
Different ways to measure the concentration of a solution, including mole fraction.
Clarification of the terms molarity and molality, and their differences in measuring solution concentration.
Critique of molarity due to its dependence on volume, which can change with pressure and temperature.
Advantages of molality as a measure of concentration that remains constant regardless of pressure and temperature changes.
Challenge to the audience to come up with mnemonics to remember the difference between molality and molarity.
Transcripts
Browse More Related Video

Solution, Suspension and Colloid | Is Matter around us pure? | Chemistry | Khan Academy

Concentration and Molarity: The Key to Chemical Solutions
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration Problems

Mixtures - Class 9 Tutorial

Solution, Suspension and Colloid | Chemistry

Solution, Suspension and Colloid | #aumsum #kids #science #education #children
5.0 / 5 (0 votes)
Thanks for rating: