9. Periodic Table; Ionic and Covalent Bonds
TLDRThis video script from an MIT OpenCourseWare lecture delves into the concepts of electron affinity and electronegativity, highlighting their significance in chemistry. Professor Catherine Drennan explains how these properties influence atomic behavior and bonding, using the periodic table to illustrate trends. The lecture also explores the role of electronegativity in pharmaceuticals, the stability of molecules in the body, and the importance of atomic and ionic radii in biological ion channels. The script concludes with an introduction to chemical bonding, including ionic, covalent, and polar covalent bonds, emphasizing their impact on molecule properties and stability.
Takeaways
- 📚 The lecture discusses electron affinity and electronegativity, highlighting their significance in chemistry and their relationship to each other.
- 🔬 Robert Millikan, an MIT alumnus, contributed to the understanding of electronegativity through an equation that helped conceptualize it better.
- 🏆 The importance of electronegativity is underscored by its role in pharmaceutical molecules, with a specific example of how it contributes to the antibiotic effect.
- 💊 The use of electronegative atoms like halogens in drug molecules is common due to their ability to stabilize the molecule and make it harder to oxidize, thus extending their effectiveness in the body.
- 🔭 The concept of atomic and ionic radii is introduced, with trends across the periodic table and the effects of ionization on these radii.
- ⚛️ The functionality of ion channels in biology, particularly in neurons and muscle cells, is tied to the size and selectivity of ions, emphasizing the importance of ionic radius.
- 🌐 The idea of isoelectronic species is presented, where different atoms or ions have the same electron configuration but may vary in size.
- 🔗 The lecture covers different types of chemical bonds, including ionic, covalent, and polar covalent bonds, explaining the forces and interactions that lead to their formation.
- 🔑 The significance of bond strength is conveyed through the dissociation energy, which is the energy required to break a bond, indicating the stability of a compound.
- 📉 The script uses a graphical representation of energy versus internuclear distance to illustrate bond formation and the concept of bond length.
- 🌡 The polarity of molecules is discussed, showing how the shape of a molecule, in addition to the presence of polar bonds, determines its overall polarity.
Q & A
What is the main topic discussed in the provided script?
-The main topic discussed in the script is electronegativity, its relation to electron affinity, and the significance of electronegativity in chemistry, particularly in the context of pharmaceutical molecules and the design of new drugs.
Why do chemists prefer to talk about electronegativity rather than electron affinity?
-Chemists prefer to talk about electronegativity because it is a more comprehensive concept that includes the ability of an atom to attract electrons from other atoms, which is highly related to but distinct from electron affinity.
Who is credited with initiating the concept of electronegativity as a way of thinking about atoms?
-Linus Pauling is credited with initiating the concept of electronegativity as a way of thinking about atoms.
What role did Robert Millikan play in relation to the concept of electronegativity?
-Robert Millikan helped refine the understanding of electronegativity by coming up with an equation that helped people think better about what electronegativity is, after Linus Pauling introduced the concept.
How is the electronegativity of an atom related to its ionization energy and electron affinity?
-Electronegativity is proportional to half of the sum of the ionization energy (IE) and the electron affinity (EA) of an atom. This relationship indicates that atoms with high electronegativity tend to attract electrons more strongly.
What is the significance of electronegativity in the pharmaceutical industry?
-Electronegativity is significant in the pharmaceutical industry because atoms that are electronegative, such as those found in many drugs, can give special properties to pharmaceutical molecules, including increased reactivity and stability in the body.
Why are halogens often added to pharmaceutical molecules?
-Halogens are often added to pharmaceutical molecules because their high electronegativity increases the reactivity of the molecule and can make the molecule more stable in the body by making it harder to oxidize, thus potentially extending the duration of the drug's effect.
What is the importance of atomic and ionic radius in the context of ion channels in biological systems?
-The atomic and ionic radius is important in the context of ion channels because these channels are highly selective for certain ions based on their size and charge, which is crucial for regulating the influx of ions into cells and allowing for rapid responses in biological systems like neurons and muscle cells.
What is the concept of isoelectronic species in chemistry?
-Isoelectronic species are atoms or molecules that have the same electron configuration, even though they may not have the same size or charge. They often exhibit similar chemical properties due to their identical electron arrangements.
How do the concepts of ionization energy and electron affinity relate to the formation of ionic bonds?
-The formation of ionic bonds involves the ionization energy, which is the energy required to remove an electron from a neutral atom to form a cation, and the electron affinity, which is the energy change associated with the gain of an electron by a neutral atom to form an anion. The overall process results in the formation of a stable ionic compound due to the electrostatic attraction between the oppositely charged ions.
What is the significance of the dissociation energy in understanding chemical bonds?
-The dissociation energy represents the amount of energy required to break a chemical bond. It is a measure of the bond's strength; a higher dissociation energy indicates a stronger bond that is more difficult to break, while a lower dissociation energy indicates a weaker bond.
Outlines
📚 Introduction to Electron Affinity and Electronegativity
The script introduces the concept of electron affinity and its relation to electronegativity, emphasizing that while electron affinity is the ability of an atom to attract an electron, chemists often focus on electronegativity, which is the ability of an atom to attract electrons from another atom. The lecture mentions the contributions of Linus Pauling and Robert Millikan to the understanding of electronegativity and its significance in the field of chemistry. It also touches on the potential of students to contribute to chemistry, much like the MIT alumni who have made significant strides in the field.
🌐 Electronegativity and its Impact on Pharmaceutical Molecules
This paragraph delves into the importance of electronegativity, especially in the context of pharmaceutical molecules. It explains how electronegative atoms, such as those found in halogenated compounds, are used in drugs due to their special properties. The script features a video testimonial from Kateryna Kozyrytska, discussing her research on microorganisms and the role of electronegativity in the effectiveness of antibiotics. The summary highlights the use of halogens in various drugs and the benefits of these electronegative atoms in enhancing the stability and reactivity of pharmaceutical compounds.
🔬 Trends in Atomic and Ionic Radii
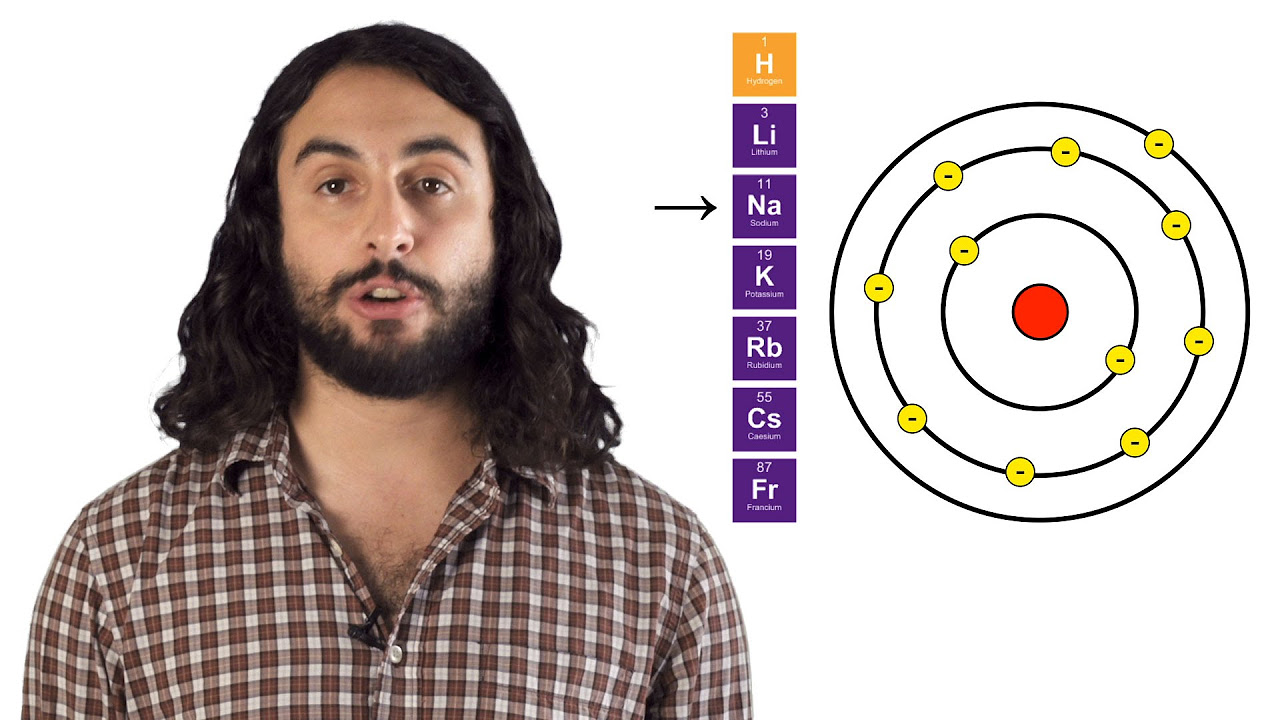
The script discusses the concept of atomic radius, defined by the distance at which the electron density is about 90% of the total. It outlines the trends in atomic radius across the periodic table, noting that an increase in effective nuclear charge (Z effective) leads to a decrease in atomic radius, while an increase in the principal quantum number (n) leads to an increase in atomic radius. The paragraph also addresses the differences in size between ions and their neutral parents, explaining that cations are smaller and anions are larger than their neutral atoms due to the loss or gain of electrons.
🚀 The Relevance of Ionic Radii in Ion Channels
This section of the script highlights the importance of ionic radii in the functionality of ion channels, which play a crucial role in biological processes such as muscle movement and neural activity. It emphasizes the selectivity of ion channels for specific ions, which is critical for maintaining proper cellular function. The script also discusses the Nobel Prize-winning work of Rod MacKinnon in determining the structures of these ion channels and the significance of the precise fit of ions within these channels for their proper function.
🔍 Exploring Isoelectronic Atoms and Their Properties
The script introduces the concept of isoelectronic atoms, which share the same electron configuration but may have different sizes due to their ionic states. It provides examples of isoelectronic species, such as fluoride, oxide, and nitride ions, which have gained electrons to achieve the electron configuration of the noble gas neon. The summary explains how the gain or loss of electrons affects the size of the ions compared to their neutral state and invites the audience to consider the implications of these differences in various chemical contexts.
🐕 Fun with Dogs Teaching Chemistry: Chemical Bonding Basics
In a lighter segment, the script presents a humorous and engaging video titled 'Dogs Teaching Chemistry!' where dogs explain the fundamentals of chemical bonding. The video covers the nature of chemical bonds, the role of valence electrons, and the distinction between ionic and covalent bonds. It also touches on polar covalent bonds, where the sharing of electrons is unequal due to differences in electronegativity between atoms. The summary captures the essence of the video, highlighting the educational yet entertaining approach to teaching chemistry.
🔬 Deep Dive into Ionic Bonds and Their Formation
This paragraph provides a detailed examination of ionic bonds, focusing on the process of ion formation through ionization energy and electron affinity. It explains the energy changes associated with the formation of cations and anions from neutral atoms and the subsequent attraction between these oppositely charged ions. The script uses the example of sodium chloride (NaCl) to illustrate the concept and includes a calculation based on Coulomb's law to determine the energy of interaction between the ions. The summary elucidates the principles behind ionic bonding and the factors contributing to the stability of ionic compounds.
🔍 Understanding the Limitations of the Ionic Model
The script acknowledges the discrepancies between the theoretical predictions of the ionic model and experimental measurements of bond energies. It discusses the oversimplifications in the model, such as the neglect of repulsive interactions between protons and electrons, which can lead to an overestimation of bond strength. The summary points out the importance of considering these factors for a more accurate understanding of ionic bonds and their associated energies.
📉 Visualizing Chemical Bonding Through Energy vs. Distance Plots
This section of the script introduces a graphical representation of chemical bonding, specifically focusing on the energy changes that occur as a function of the distance between two atoms. It uses the example of hydrogen (H2) to illustrate the concept of bond dissociation energy and explains how the plot can be used to determine bond strength and length. The summary emphasizes the importance of understanding these energy relationships for analyzing the stability and properties of chemical bonds.
🔬 Comparing Bond Strengths and Lengths in Different Molecules
The script compares the bond strengths and lengths of different molecules, such as hydrazine and molecular nitrogen (N2), using the energy versus distance plot. It explains how the depth of the energy well on the plot corresponds to the bond dissociation energy and how the position of the minimum energy point corresponds to the bond length. The summary highlights the insights that can be gained from these plots regarding the relative stability and lengths of chemical bonds in various compounds.
🌐 The Significance of Polar Covalent Bonds in Molecular Polarity
This paragraph explores the concept of polar covalent bonds, which result from the unequal sharing of electrons between atoms with different electronegativities. It discusses the criteria for classifying a bond as polar covalent and the implications of these bonds for the overall polarity of a molecule. The script uses examples such as carbon dioxide and water to illustrate how the molecular shape can influence the cancellation of bond dipoles and result in either polar or non-polar molecules. The summary underscores the importance of considering both electronegativity differences and molecular geometry when assessing molecular polarity.
💊 Polar Bonds and Their Role in Vitamin Solubility
The script examines the role of polar bonds in determining the solubility of vitamins, specifically contrasting vitamin A and folic acid (vitamin B-9). It explains how the number of polar bonds in a molecule affects its solubility in water or fat, and the implications this has for vitamin supplementation and potential toxicity. The summary highlights the importance of understanding the relationship between molecular polarity and solubility for proper vitamin intake and health.
Mindmap
Keywords
💡Electron Affinity
💡Electronegativity
💡Ionization Energy
💡Electron Acceptor
💡Periodic Table
💡Pharmaceutical Molecules
💡Halogens
💡Atomic Radius
💡Ion Channels
💡Isoelectronic
💡Chemical Bonds
Highlights
Electron affinity and electronegativity are highly related concepts, with electronegativity being more commonly discussed in chemistry.
Electronegativity was conceptualized by Linus Pauling and later quantified by Robert Millikan, who also has a connection to MIT as an undergraduate.
Electronegativity is proportional to the square root of the sum of ionization energy and electron affinity, indicating an atom's ability to attract electrons.
Atoms with high electronegativity tend to be electron acceptors, while those with low electronegativity are more likely to be electron donors.
Electronegativity is crucial in understanding the properties of atoms used in pharmaceutical molecules, such as their reactivity and stability.
The video featuring Kateryna Kozyrytska discusses the role of electronegativity in the development of antibiotics and the study of microorganisms.
The addition of halogens to molecules is a significant practice in pharmaceuticals, as it can enhance the stability and effectiveness of drugs.
Halogenation can make a molecule more electron poor, which can reduce its susceptibility to oxidation and increase its stability in the body.
Atomic radius is defined by the distance at which the electron density is approximately 90% of the total, indicating the size of an atom.
Trends in atomic radius across the periodic table are influenced by the effective nuclear charge and principal quantum number.
Ion channels play a vital role in biological processes, such as muscle movement and neural signaling, by regulating the flow of ions.
Ion channels are highly selective for specific ions, which is crucial for maintaining the proper balance of elements within cells.
The concept of isoelectronic species, which share the same electron configuration, is introduced, affecting their size and properties.
Chemical bonding is explained through the lens of dogs in a creative video, simplifying the concepts of ionic, covalent, and polar covalent bonds.
The formation of ionic bonds, such as in NaCl, involves the transfer of electrons and the creation of cations and anions that attract each other.
Covalent bonds involve the sharing of a pair of valence electrons between two atoms, leading to the formation of molecules.
Polar covalent bonds are characterized by an unequal sharing of electron pairs due to a significant difference in electronegativity between the atoms involved.
The stability and strength of a chemical bond can be inferred from its dissociation energy and bond length, which are derived from energy versus distance plots.
Molecular polarity is influenced by both the presence of polar bonds and the three-dimensional shape of the molecule, as demonstrated by the examples of CO2 and H2O.
The number of polar bonds in a molecule can be an indicator of its polarity and its solubility properties, such as in the case of vitamins A and B-9 (Folic Acid).
Transcripts
Browse More Related Video

BTEC Applied Science: Unit 1 Chemistry Covalent Bonds

[H2 Chemistry] 2023 Topic 1 Atomic Structure & Physical Periodicity 2

Mastering Chemical Bonding: Explained with 3D Animation

Introduction to Ionic Bonding and Covalent Bonding

[H2 Chemistry] 2023 Topic 2 Chemical Bonding 2
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
5.0 / 5 (0 votes)
Thanks for rating: