Chem 51A 11/09/09 Ch. 6. Introduction to Understanding Organic Reactions
TLDRChapter 6 introduces the foundational concepts of organic chemistry, focusing on substitution and elimination reactions. It covers reaction mechanisms, energetics, and rate-determining steps. The chapter prepares students for more complex topics in chapters 7 and 8, including stereochemistry and detailed reaction mechanisms. Key concepts such as the use of curved arrows to show electron flow and the role of solvents in reactions are discussed. The lecture also touches on addition reactions, reactive intermediates, and the different types of arrows used in organic chemistry to represent reactions and equilibria.
Takeaways
- 🔬 Chapter 6 introduces reactivity and reaction mechanisms, laying the groundwork for substitution and elimination reactions.
- 📚 The course will focus on understanding reaction mechanisms, including the use of curved arrows to illustrate electron flow.
- ⚛️ Types of reactions discussed include substitution, elimination, and addition reactions.
- ⚡ Energetics and rates of reactions are important concepts, including rate-determining steps.
- 🧪 Solvents play a crucial role in reactions, allowing reactants to mix, stabilizing ions, and dissipating heat.
- 🔄 Various arrows are used to represent different aspects of reactions: single arrows for reactions, double-headed arrows for equilibrium, and curved arrows for electron flow.
- 🧲 Homolytic cleavage involves breaking bonds with single electrons, indicated by fish hook arrows, while heterolytic cleavage involves electron pairs.
- 🧬 Resonance structures are represented with double-headed arrows, indicating that the molecule is both structures simultaneously.
- ➕ Addition reactions involve breaking a pi bond and forming two new sigma bonds, such as adding bromine to an alkene.
- ➖ Elimination reactions are the reverse of addition reactions, breaking two sigma bonds to form a new pi bond.
- ⚛️ Reactive intermediates include carbocations, free radicals, and carbanions, each with unique properties and reactivities.
Q & A
What is the main focus of Chapter 6 in the lecture?
-Chapter 6 focuses on reactivity and how chemical reactions occur, setting the groundwork for understanding reaction mechanisms, particularly substitution and elimination reactions.
What are the two types of reactions introduced in Chapter 6?
-Chapter 6 introduces substitution reactions and addition reactions.
Why are solvents important in organic reactions?
-Solvents allow reactants to mix, stabilize ions, dissipate heat from exothermic reactions, and prevent explosions by allowing reactions to be carried out at controlled temperatures.
What is the role of curved arrows in organic chemistry?
-Curved arrows are used to show the flow of electrons during the making and breaking of bonds in reaction mechanisms.
What is an example of a substitution reaction provided in the lecture?
-An example given is the reaction of ethyl iodide (iodoethane) with sodium cyanide, where cyanide replaces iodine to form a nitrile group.
How are addition reactions characterized?
-Addition reactions involve breaking a pi bond and forming two new sigma bonds, as illustrated by the reaction of cyclohexene with bromine.
What is an example of an elimination reaction?
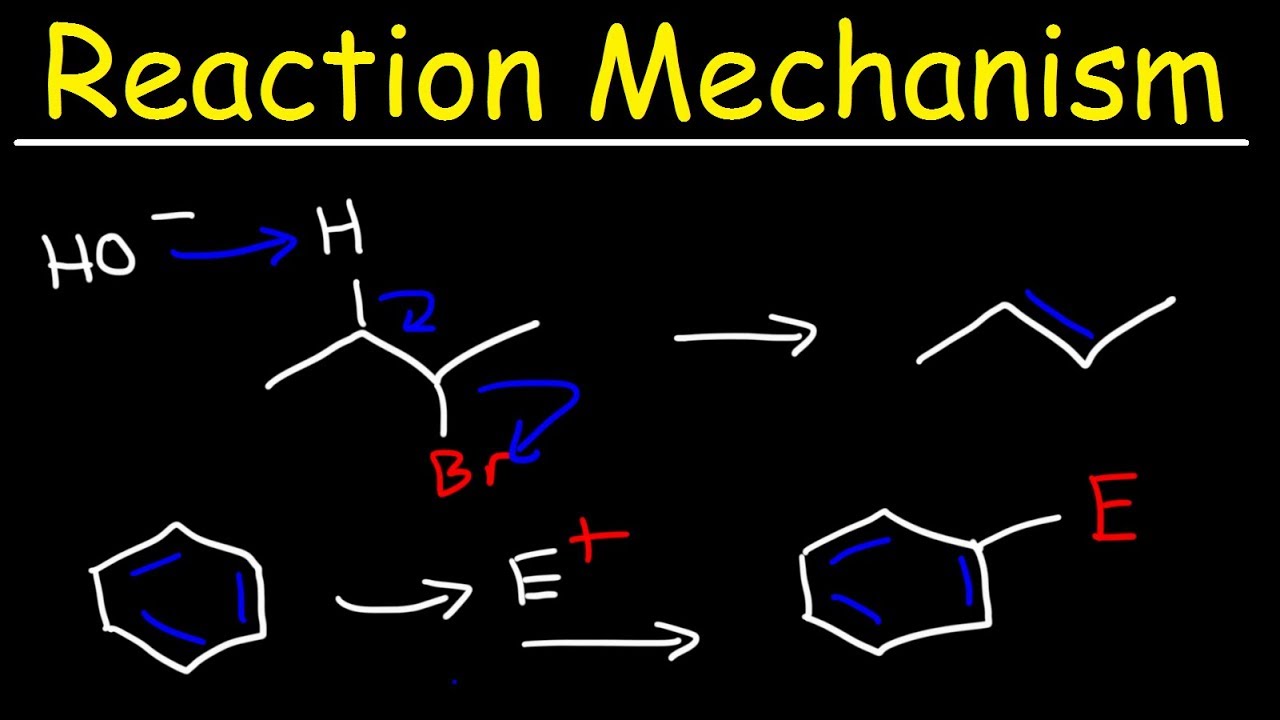
-An example of an elimination reaction is the reaction of tert-butyl bromide with sodium ethoxide in ethanol, forming isobutene (2-methylpropene).
What are reactive intermediates?
-Reactive intermediates are transient species formed during reactions, such as carbocations, free radicals, and carbanions.
How do carbocations and free radicals differ in their reactions?
-Carbocations seek two electrons to complete their octet and tend to share electrons, while free radicals seek one electron and often involve reactions that result in stealing an electron.
What type of reaction mechanism is illustrated by the protonation of tert-butyl alcohol?
-The protonation of tert-butyl alcohol followed by substitution with bromine is an example of a reaction that proceeds through a carbocation intermediate, specifically an SN1 reaction.
Outlines
📘 Introduction to Chapter 6 and Reactivity Concepts
Chapter 6 serves as a foundation for understanding reactivity and chemical reactions. It introduces the concepts of substitution and elimination reactions, emphasizing the importance of understanding reaction mechanisms, energy barriers, and reaction rates. The chapter also discusses the significance of curved arrows in depicting chemical processes and prepares students for the upcoming content in Chapters 7 and 8.
🔬 Example of Organic Reaction: Ethyl Iodide with Sodium Cyanide
The reaction of ethyl iodide with sodium cyanide is examined. Various ways of representing the reaction in organic chemistry are discussed, highlighting the role of solvents in facilitating reactions. Solvents help mix reactants, stabilize ions, and dissipate heat, which is crucial for safely conducting exothermic reactions. The different notations for representing organic reactions are also explained.
🔄 Substitution Reaction Mechanisms
The concept of substitution reactions is explored in detail. The example of replacing iodine in ethyl iodide with a cyanide group is used to illustrate the process. The importance of using correct arrows to represent the movement of electrons in chemical reactions is emphasized. Different types of arrows, including those indicating equilibrium and electron pair movement, are discussed.
🔁 Understanding Equilibrium Arrows and Electron Flow
The use of arrows to indicate chemical equilibria and electron flow in reactions is explained. Different types of arrows and their meanings are discussed, including those used to show reversible reactions and the flow of electron pairs. The significance of correct arrow usage in organic chemistry is highlighted to avoid miscommunication.
⚡ Curved Arrows and Radical Reactions
The use of curved arrows to show the flow of electron pairs in reactions is explained. The example of an SN2 displacement reaction is used to illustrate this concept. The text also introduces single electron movements using 'fish hook' arrows, particularly in reactions involving free radicals, such as the breaking of peroxide bonds under heat.
💥 Free Radicals and Homolytic Cleavage
The concept of free radicals and their formation through homolytic cleavage is discussed. The example of breaking peroxide bonds to form methoxy radicals is used to illustrate this. The differences between bond strengths in homolytic and heterolytic cleavages are explained, with a focus on the energy dynamics involved in these processes.
🔗 Addition Reactions: Breaking and Forming Bonds
Addition reactions, where elements are added across a pi bond resulting in the formation of two new sigma bonds, are discussed. The example of cyclohexene reacting with bromine is used to illustrate this type of reaction. The basic mechanism of addition reactions and their significance in organic chemistry are explained.
➖ Elimination Reactions: Forming Pi Bonds
Elimination reactions, the reverse of addition reactions, are explored. These reactions involve breaking two sigma bonds to form a new pi bond. The example of tert-butyl bromide reacting with sodium ethoxide to form 2-methylpropene is used to illustrate the concept. The significance of these reactions in understanding organic mechanisms is emphasized.
⚛️ Reactive Intermediates: Carbo Cations and Radicals
The concept of reactive intermediates, including carbo cations, radicals, and carbon ions, is introduced. The transient nature of these intermediates and their roles in organic reactions are explained. The differences in reactivity and stability of these intermediates are discussed, with a focus on their formation and reaction mechanisms.
🔄 Substitution Reactions with Reactive Intermediates
An example of a substitution reaction involving a reactive intermediate is provided. The step-by-step mechanism of this reaction, which involves protonating an oxygen atom and forming a tert-butyl carbocation, is detailed. The reaction's progression to the final product through the interaction with bromine anion is explained. The importance of understanding such mechanisms in organic chemistry is emphasized.
Mindmap
Keywords
💡Substitution Reactions
💡Elimination Reactions
💡Reaction Mechanisms
💡Curved Arrows
💡Energetics
💡Rate Determining Step
💡Reactive Intermediates
💡Addition Reactions
💡Homolytic Cleavage
💡Resonance Structures
Highlights
Chapter 6 introduces the concept of reaction mechanisms, focusing on reactivity and how chemical reactions occur, with an emphasis on substitution and elimination reactions.
Understanding reaction mechanisms involves learning about energetics, the rates of reactions, and rate-determining steps.
Organic reactions are often carried out in solvents to allow reactants to mix, stabilize ions, and dissipate heat, preventing potential explosions.
Different ways of writing chemical reactions are explored, emphasizing the importance of understanding the context and components involved.
Substitution reactions involve the replacement of one group by another, exemplified by the reaction of ethyl iodide with sodium cyanide.
The use of curved arrows to indicate the flow of electrons is integral to understanding reaction mechanisms in organic chemistry.
Radical reactions, which involve single electrons, are introduced, including the concept of fish hook arrows to show electron movement.
The distinction between homolytic and heterolytic bond cleavage is explained, highlighting the different energy requirements and resulting intermediates.
Resonance structures and the use of double-headed arrows to represent them are discussed, clarifying the concept of delocalized electrons.
Addition reactions, which involve breaking a pi bond and forming new sigma bonds, are introduced with examples such as the reaction of cyclohexene with bromine.
The concept of paired opposite reactions is explored, such as the relationship between addition and elimination reactions.
Elimination reactions involve breaking two sigma bonds to form a new pi bond, as demonstrated by the reaction of tert-butyl bromide with sodium ethoxide.
Reactive intermediates, including carbo cations, free radicals, and carbanions, are introduced as key species in understanding organic reaction mechanisms.
The lecture emphasizes the importance of recognizing concerted processes versus stepwise processes in reaction mechanisms.
An example of a stepwise substitution reaction is provided, illustrating the formation of a reactive intermediate and its subsequent reaction to form the final product.
Transcripts
Browse More Related Video

Mechanisms | Explained | Year 12 or AS Chemistry | Organic Chemistry | A level Chemistry

Lec-08 I Types of organic reactions I Applied Chemistry I Chemical engineering

Intro to Reaction Mechanisms: Crash Course Organic Chemistry #13
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement

Organic Chemistry 1 Exam 2 Review Questions

6.5 Curved Arrow Pushing in Reaction Mechanisms | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: