Atoms & the Periodic Table (old version)
TLDRThis educational video script delves into the fundamentals of atoms and the periodic table, explaining the structure of an atom with its nucleus containing protons and neutrons, and electrons orbiting in an electron cloud. It clarifies the difference between elements and atoms, using aluminum as an example. The script also teaches how to read the periodic table, identifying atomic numbers and masses, and explains the concept of electron stability across different energy levels. It further explores chemical bonds, distinguishing between covalent bonds, where atoms share electrons, and ionic bonds, where electrons are transferred to form charged ions that attract each other, exemplified by the formation of salt from sodium and chlorine.
Takeaways
- 🌌 An atom is the smallest unit of matter and the building block of the universe, consisting of protons, neutrons, and electrons.
- 🔴 The nucleus of an atom contains protons, which are positively charged, and neutrons, which are neutral (zero charge).
- ⚫ Electrons orbit the nucleus in an electron cloud and carry a negative electrical charge.
- ⚖️ Atoms typically have an equal number of protons and electrons, maintaining electrical neutrality.
- 📊 The periodic table of elements is a catalog of all known elements, each made up of atoms of a single type.
- 🔢 The atomic number signifies the number of protons in an atom, and is usually equal to the number of electrons.
- 📚 The atomic mass or weight represents the sum of protons and neutrons in an atom's nucleus.
- 🪐 Electrons occupy energy levels or shells around the nucleus, with the first level holding up to two electrons and subsequent levels holding up to eight each.
- 💥 Atoms are considered stable when their outermost electron level is full. Unstable atoms tend to form molecules through chemical bonds.
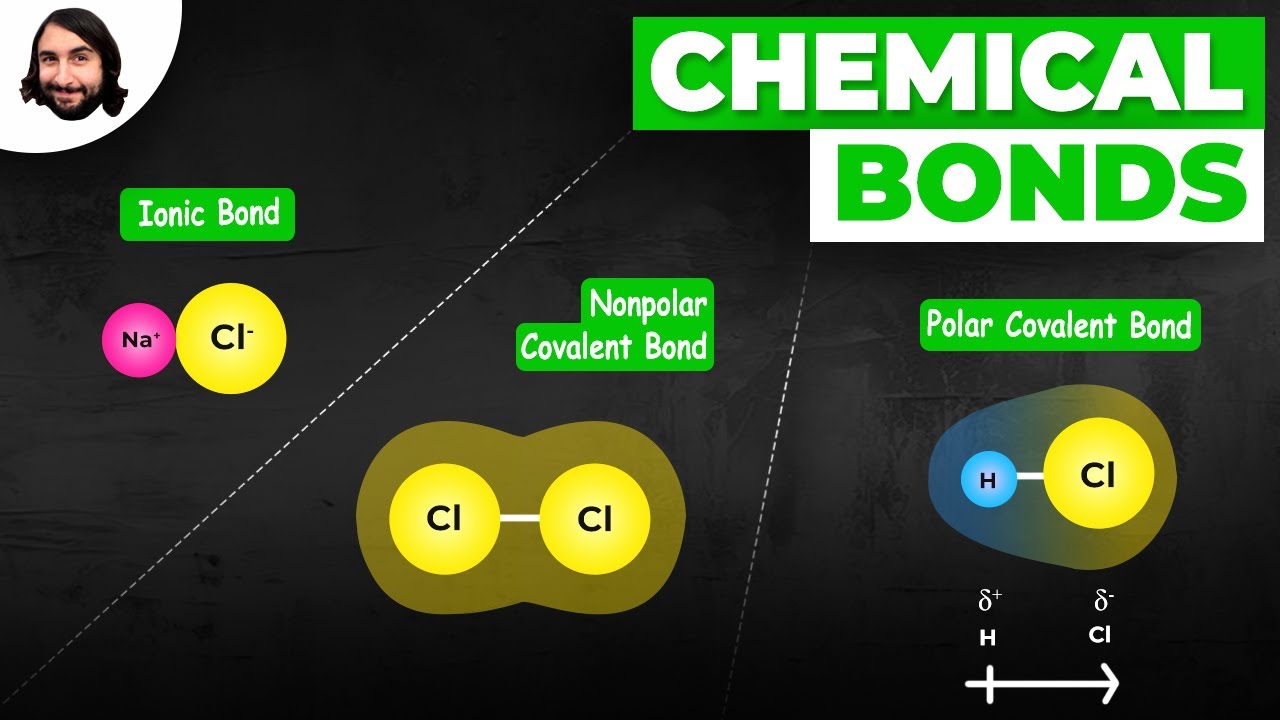
- 🔗 Covalent bonds occur when atoms share electrons to achieve stability, as seen in the molecule O2 (oxygen gas).
- ⚡ Ionic bonds form when one atom gains or loses electrons, creating charged ions that are attracted to each other, as in the formation of table salt (sodium chloride).
Q & A
What is an atom?
-An atom is the smallest unit of matter that makes up everything in our known universe. It consists of a nucleus containing protons and neutrons, with electrons orbiting around the nucleus in an electron cloud.
What are the three main parts of an atom?
-The three main parts of an atom are the nucleus, which contains protons and neutrons, and the electron cloud, where electrons orbit the nucleus.
What is the charge of a proton, and what is its role in an atom?
-A proton has a positive charge and is located in the nucleus of an atom, contributing to the atomic number and the overall positive charge of the nucleus.
What is the atomic number, and how is it determined?
-The atomic number is the number of protons in an atom's nucleus, which defines the element and is usually equal to the number of electrons in a neutral atom.
What is the difference between an element and an atom?
-An element is a pure substance made from only one type of atom, while an atom is the smallest unit of that element. For example, a sheet of aluminum foil is made from the element aluminum, which consists of billions of aluminum atoms.
What does the atomic mass represent, and how is it calculated?
-The atomic mass, or atomic weight, represents the total number of protons and neutrons in an atom's nucleus. It is calculated by adding the number of protons to the number of neutrons.
Why are electrons considered to have negligible impact on an atom's mass?
-Electrons have a negligible impact on an atom's mass because they are much smaller and lighter compared to protons and neutrons. Their mass is so small that it does not significantly affect the overall atomic mass, similar to the weight difference before and after a haircut.
What are the two main types of chemical bonds that can form between atoms?
-The two main types of chemical bonds are covalent bonds, where atoms share electrons, and ionic bonds, where electrons are transferred from one atom to another, resulting in charged ions that are attracted to each other.
How do atoms achieve stability in their electron configuration?
-Atoms achieve stability when their outermost electron level is full, typically with eight electrons (except for the first level, which can only hold two). This fullness allows the atom to be stable and less likely to participate in chemical reactions.
What is a covalent bond, and how is it formed?
-A covalent bond is a chemical bond formed when atoms share electrons to achieve a stable electron configuration. This often occurs between non-metal atoms, allowing them to fill their outer electron levels.
What is an ionic bond, and how does it differ from a covalent bond?
-An ionic bond is a chemical bond formed when one atom transfers electrons to another atom, resulting in positively and negatively charged ions that are attracted to each other. This is different from a covalent bond, where electrons are shared rather than transferred.
Why do oxygen atoms form a double bond when they combine to form a molecule?
-Oxygen atoms form a double bond because each oxygen atom needs two more electrons to fill its outer electron level and achieve stability. By sharing two pairs of electrons, both oxygen atoms can reach a stable configuration with eight electrons in their outer level.
How does the process of ionic bonding lead to the formation of salt?
-Ionic bonding leads to the formation of salt when sodium (Na), which has one electron in its outer level, loses that electron to chlorine (Cl), which needs one more electron to fill its outer level. Sodium becomes positively charged, and chlorine becomes negatively charged, forming an ionic bond and creating sodium chloride (NaCl), or salt.
Outlines
🌌 Introduction to Atoms and the Periodic Table
This paragraph introduces the fundamental concept of atoms, which are the smallest units of matter that constitute the universe. It explains the basic structure of an atom, highlighting the nucleus containing protons and neutrons, and the electrons orbiting in an electron cloud. The paragraph also distinguishes between an atom and an element, using aluminum foil as an example to illustrate that a macroscopic material is made up of many atoms of the same element. Additionally, it introduces the periodic table as a catalog of known elements and explains how to read the atomic number and atomic mass from the table, which represent the number of protons and the total number of protons and neutrons, respectively.
🔍 Understanding Atomic Structure and the Periodic Table
The second paragraph delves deeper into the atomic structure, explaining how to determine the number of protons, electrons, and neutrons from the periodic table. It uses beryllium as an example to demonstrate the calculation process. The paragraph clarifies that the atomic number corresponds to the number of protons, which is usually equal to the number of electrons, and the atomic mass is the sum of protons and neutrons. It also touches on the concept of electron energy levels, explaining that the first energy level can hold up to two electrons, making it stable when full.
🔬 Electron Configuration and Atomic Stability
This paragraph continues the discussion on electron energy levels, explaining that the second energy level can hold up to eight electrons for stability. It uses helium, neon, and argon as examples to illustrate atoms with full energy levels, which are considered stable. The paragraph then introduces the concept of atomic instability when the outermost electron level is not full, as exemplified by carbon, which has only four electrons in its second level, requiring four more to be stable. The paragraph sets the stage for discussing chemical bonds, which form when atoms are not stable and seek to achieve stability by sharing or transferring electrons.
💧 Covalent Bonds and Molecular Formation
The fourth paragraph focuses on covalent bonds, where atoms share electrons to achieve stability. It uses the molecule of oxygen (O2) as a prime example, explaining that each oxygen atom shares two electrons with another oxygen atom, resulting in a stable molecule with eight electrons in each atom's outer level. The paragraph also describes the process of electron sharing visually, likening it to an 8-shaped motion between the two atoms, and explains that this sharing results in a double bond, represented by two dashes in chemical notation.
⚛️ Ionic Bonds and the Formation of Salt
The final paragraph introduces ionic bonds, which occur when one atom gains or loses electrons, creating charged particles known as ions. It contrasts this with covalent bonds, where electrons are shared. The paragraph uses the example of sodium chloride (table salt) to explain how sodium, with one electron in its outer level, loses that electron to chlorine, which needs one more electron to fill its outer level. This transfer results in a positively charged sodium ion and a negatively charged chloride ion, which are attracted to each other due to their opposite charges, forming an ionic bond. The paragraph concludes with a brief explanation of how the loss and gain of electrons change the atomic structure and net charge of the atoms involved.
Mindmap
Keywords
💡Atom
💡Nucleus
💡Electron
💡Periodic Table
💡Atomic Number
💡Atomic Mass
💡Electron Cloud
💡Energy Levels
💡Covalent Bond
💡Ionic Bond
💡Ion
Highlights
An atom is the smallest unit of matter and consists of protons, neutrons, and electrons.
Protons are positively charged, neutrons are neutral, and electrons carry a negative charge.
The number of protons usually equals the number of electrons in an atom.
The periodic table organizes elements based on the number of protons, known as the atomic number.
Elements are pure substances made of only one type of atom, differing from atoms in scale and composition.
The atomic mass represents the sum of protons and neutrons in an atom's nucleus.
Electrons are too light to significantly affect an atom's mass, similar to the negligible weight of hair before and after a haircut.
The atomic number defines the number of protons and usually matches the number of electrons.
Electrons occupy energy levels or shells around the nucleus, with the first level holding a maximum of two electrons.
An atom is stable when its outermost electron level is full.
Unstable atoms with incomplete outer electron levels tend to form molecules through chemical bonds.
Covalent bonds involve atoms sharing electrons to achieve stability, as seen in the O2 molecule.
Ionic bonds occur when one atom transfers electrons to another, creating charged ions that attract each other.
Sodium chloride (table salt) is an example of an ionic bond, formed by the transfer of an electron from sodium to chlorine.
The net charge of an atom is the balance between protons and electrons, which becomes zero in neutral atoms.
Ions are atoms with a net charge due to the loss or gain of electrons, leading to ionic bonding.
Magnetic attraction between oppositely charged ions results in the formation of ionic compounds.
Transcripts
Browse More Related Video

Atoms and Molecules (ionic vs covalent bonds)

Introduction to Ionic Bonding and Covalent Bonding

Polar and Nonpolar Covalent Bonds

What Is An Atom - Part 1 | Properties of Matter | Chemistry | FuseSchool

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
5.0 / 5 (0 votes)
Thanks for rating: