The Most Misunderstood Concept in Physics
TLDRThe video explores the concept of entropy, which governs everything from molecular collisions to the evolution of the universe. It traces the history of the discovery of entropy, from French physicist Sadi Carnot's development of an ideal heat engine in the 1800s to Ludwig Boltzmann and Rudolf Clausius formulating the laws of thermodynamics. The script explains how entropy drives the arrow of time, determines efficiency limits for heat engines, enables phenomena like air conditioning, and may have even given rise to life on Earth. Ultimately entropy leads the universe inexorably towards uniformity and its inevitable 'heat death', when no processes can occur.
Takeaways
- 😲 Entropy governs many natural processes - from molecular collisions to evolution of the universe.
- 😎 Sadi Carnot analyzed ideal heat engines and developed concepts that led to the second law of thermodynamics.
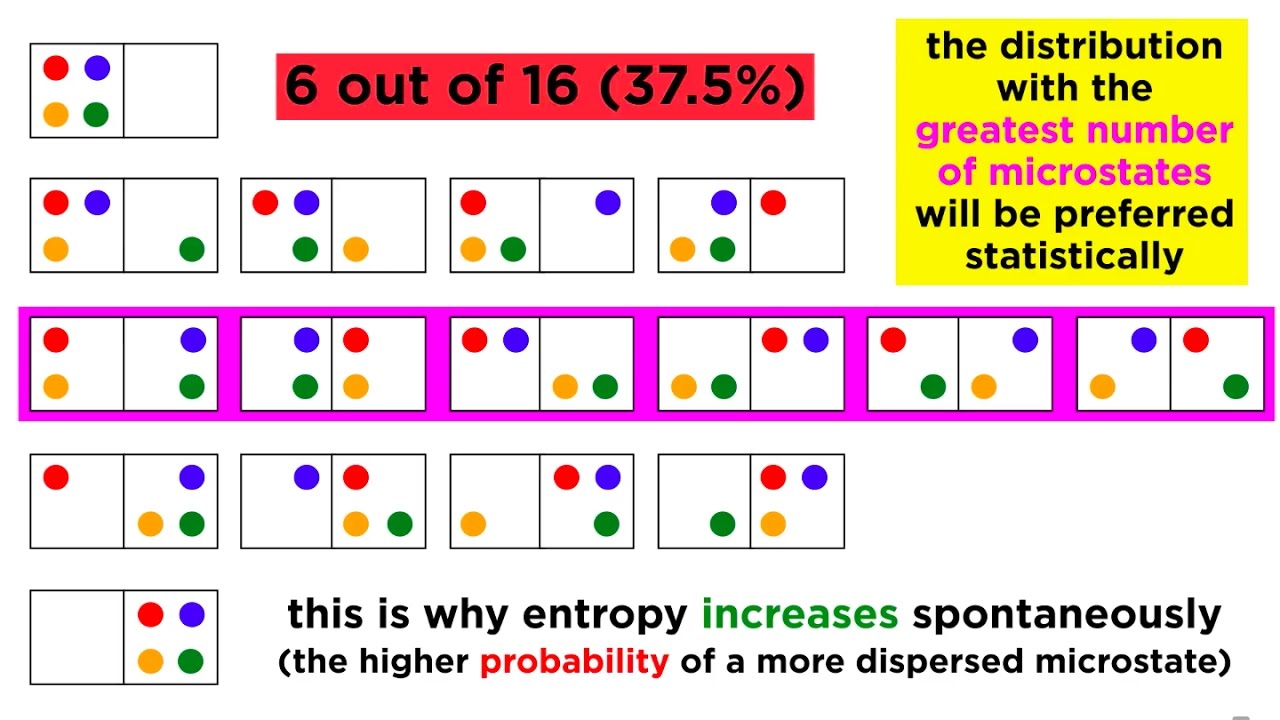
- 🤓 Entropy is a measure of how spread out energy is - low entropy means concentrated, high entropy means dispersed.
- 🔬 The second law states that entropy of the universe tends towards a maximum over time.
- 🌡 Efficiency of a heat engine depends on temperatures of the hot and cold reservoirs.
- 👀 Direction of time emerges from the fact that things evolve from unlikely to more likely states.
- ☀️ Sun provides Earth with low entropy, concentrated energy that enables growth of complexity.
- 🍵 Mixing milk in tea shows how complexity emerges at intermediate levels of entropy.
- ⏳ In the very far future, the heat death of the universe will occur when entropy is at a maximum.
- 🤯 Black holes contain vast amounts of entropy proportional to their surface area.
Q & A
What concept does the video primarily discuss?
-The video primarily discusses the concept of entropy, a fundamental principle in thermodynamics that describes the tendency of energy to spread out and the state of disorder in a system.
How does entropy relate to the direction of time according to the video?
-Entropy relates to the direction of time by providing a clear distinction between the past and the future. The increase in entropy, from unlikely to more likely states, explains why there is an arrow of time, moving in one direction towards higher entropy.
What is Sadi Carnot known for, as mentioned in the video?
-Sadi Carnot is known for his insights into heat engines, particularly his conceptualization of an ideal heat engine that operates with no friction and no losses to the environment. His work laid the foundation for understanding the efficiency of heat engines and the principles of thermodynamics.
How is the efficiency of an ideal heat engine determined?
-The efficiency of an ideal heat engine is determined by the temperatures of the hot and cold sides. It depends on the difference between the heat flowing into the engine from the hot source and the heat flowing out to the cold sink, divided by the heat input from the hot source.
Why can't a heat engine be 100% efficient, according to the principles discussed in the video?
-A heat engine can't be 100% efficient because, to return the piston to its original position, some heat must be dumped into the cold reservoir. This means not all the energy stays in the system (e.g., the flywheel), indicating that some energy becomes less usable due to entropy.
What role does the Sun play in the Earth's entropy and energy flow, as described in the video?
-The Sun plays a critical role in providing Earth with low entropy, or concentrated bundled up energy, which is more useful than the energy Earth radiates back into space. This steady stream of low entropy from the Sun enables life and various processes on Earth by allowing for a temporary decrease in entropy in localized systems while the overall entropy of the universe increases.
How does life on Earth relate to the second law of thermodynamics?
-Life on Earth is related to the second law of thermodynamics as it is exceptionally good at converting low entropy (concentrated energy) into high entropy (spread out energy). The process of life accelerates the natural tendency of the universe towards maximum entropy.
What was Ludwig Boltzmann's contribution to understanding entropy?
-Ludwig Boltzmann contributed to understanding entropy by showing that heat flowing from cold to hot is not impossible but merely improbable. He introduced the concept that the natural tendency of energy is to spread out, which is reflected in the vast number of configurations energy can take in a system.
Why is the early universe considered to have low entropy, despite being hot and dense?
-The early universe is considered to have low entropy because, taking gravity into account, having matter evenly spread out in a hot, dense state is extremely unlikely and thus represents a state of low entropy. Gravity's role in clumping matter together increases the entropy over time as the universe expands and cools.
What is the significance of black holes in relation to the universe's entropy?
-Black holes significantly contribute to the universe's entropy, as their entropy is proportional to their surface area. As black holes grow, their entropy increases, making them major contributors to the overall entropy of the universe. Stephen Hawking's discovery of Hawking radiation confirmed that black holes emit radiation and have a temperature, further supporting their role in the universe's entropy.
Outlines
😕The earth gets energy from the sun, but people don't understand what form it takes
This paragraph introduces the key question of what the Earth gets from the sun. It highlights that while people can name things like light, warmth, and vitamin D, they fail to recognize that fundamentally the Earth gets energy from the sun. The paragraph then explains that the amount of energy the Earth radiates back into space is less than what it receives from the sun.
🔬Carnot's ideal heat engine model shows efficiency depends on temperature, not design
This paragraph discusses French engineer Sadi Carnot's creation of an ideal theoretical heat engine to understand and improve steam engine efficiency. It explains how Carnot's reversible engine illustrated that efficiency depends only on the temperature of the hot and cold reservoirs, not the engine design. This discovery later led Lord Kelvin to develop an absolute temperature scale.
💡Entropy is the tendency of energy to spread out over time
This paragraph covers how German physicist Rudolf Clausius built on Carnot's work to define a new concept called entropy. Entropy measures how spread out energy is - low entropy meaning concentrated energy and high entropy meaning dispersed, less usable energy. The second law of thermodynamics states that entropy in the universe tends toward a maximum over time.
🌞Earth's decrease in entropy is powered by the Sun's low entropy
This paragraph asks how localized decreases in entropy are possible given the second law. It explains that Earth is not a closed system - the Sun provides a steady input of low entropy, concentrated energy. This powers Earth's processes and life itself, which accelerate entropy production. But the entropy decrease on Earth is smaller than the required entropy increase at the Sun.
🤯The low entropy early universe set the stage for increasing complexity
This paragraph examines how despite uniform high temperatures, the early universe had low entropy due to matter being spread out and gravity's clumping tendency. As the universe expanded and cooled, matter clumped into stars and galaxies, releasing kinetic energy, converting it to heat, and increasing entropy in the process. So the low entropy early universe enabled the rise of complexity.
⏳Black holes now contain most of the universe's entropy
This final paragraph discusses physicist Jacob Bekenstein's controversial idea that black holes have entropy proportional to their surface area. It covers how Stephen Hawking's work experimentally demonstrated this entropy. Supermassive black holes now contain nearly all the entropy in the universe - massively more than the early universe. So the huge rise of entropy over cosmic time is tied to black holes.
Mindmap
Keywords
💡Entropy
💡Carnot's Ideal Heat Engine
💡Second Law of Thermodynamics
💡Absolute Zero
💡Heat Death of the Universe
💡Thermodynamics
💡Closed System
💡Efficiency
💡Black Holes
💡Hawking Radiation
Highlights
First significant research finding
Introduction of new theoretical model
Proposal of innovative analysis method
Key takeaways and practical applications
Transcripts
Browse More Related Video
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates

This Concept In Physics Scares Scientists - It’s Not Looking Good
Carnot Engine
Carnot Heat Engines, Efficiency, Refrigerators, Pumps, Entropy, Thermodynamics - Second Law, Physics
24. The Second Law of Thermodynamics (cont.) and Entropy
How Quantum Entanglement Creates Entropy
5.0 / 5 (0 votes)
Thanks for rating: