7.1b Slater's Rules | General Chemistry
TLDRThis educational video delves into Slater's Rules, a method for refining the approximation of the screening or shielding constant in effective nuclear charge calculations. Though mainly relevant for advanced chemistry students, the lesson explains how to apply these rules to various elements, including helium, carbon, and vanadium. The video provides step-by-step examples to illustrate how Slater's Rules can alter the perceived attraction between valence electrons and the nucleus, impacting our understanding of atomic properties like ionization energy and atomic radius.
Takeaways
- 📘 Slater's Rule is used for a better approximation of the screening or shielding constant in calculating effective nuclear charge.
- 👨🎓 Most general chemistry students will not need to use Slater's Rules, but some, particularly in majors classes, may encounter them.
- 🔬 Effective nuclear charge helps explain trends in atomic radius, previously approximated as equal to the number of valence electrons.
- 🌟 Slater accounted for two factors: the incorrect assumption that electrons in the same shell don't screen each other, and the incorrect assumption that all core electrons have a screening value of one.
- 👉 Slater's Rules provide a more accurate approximation by assigning specific screening values to different electron interactions.
- 🚀 Example calculations for helium, carbon, and vanadium demonstrate the application of Slater's Rules to determine effective nuclear charge more accurately.
- 🔍 For helium, the effective nuclear charge is calculated to be 1.7, which is less than the atomic number of 2, indicating less attraction to the nucleus than initially assumed.
- 🌐 For carbon, the effective nuclear charge is 3.25, showing that the attraction of the valence electrons to the nucleus is less than the previously approximated +4.
- 🌌 Vanadium's example highlights the application of Slater's Rules to transition metals, showing different effective nuclear charges for 4s and 3d electrons.
- 💡 The effective nuclear charge for a 3d electron in vanadium is higher than for a 4s electron, which aligns with the observation that 4s electrons are removed before 3d electrons in chemical reactions.
- 📚 Chad's Prep offers various science prep courses, including MCAT, DAT, and OIT, and provides a free trial for their General Chemistry Master Course.
Q & A
What is the main purpose of Slater's rules?
-Slater's rules are used to get a better approximation of the screening or shielding constant when calculating the effective nuclear charge.
Why might some students in general chemistry not need to learn about Slater's rules?
-Most students taking general chemistry will not be required to know Slater's rules, as they might only be relevant for a certain percentage of students, possibly in more advanced classes like general chemistry for majors.
What is the significance of effective nuclear charge in understanding atomic radius?
-Effective nuclear charge helps explain the trend in atomic radius. It determines the attraction between the nucleus and the valence electrons, which in turn affects the size of the atom.
How does Slater's rules improve the approximation of effective nuclear charge compared to the previous lesson?
-Slater's rules account for the screening effect of electrons within the same shell and the varying screening effect of core electrons, leading to a more accurate approximation of the effective nuclear charge.
What is the screening value for 1s electrons when they screen each other according to Slater's rules?
-For 1s electrons screening each other, the screening value is 0.3 according to Slater's rules.
How does the screening constant for electrons in the same shell differ from what was previously thought?
-Previously, it was thought that electrons in the same shell did not screen each other, but Slater's rules show that they do, with a typical screening value of 0.35.
What is the effective nuclear charge for helium using Slater's rules?
-Using Slater's rules, the effective nuclear charge for helium is calculated to be 1.7, which is less than the previously approximated value of 2.
How does Slater's rules apply to the calculation of effective nuclear charge for a 2p electron in carbon?
-For a 2p electron in carbon, Slater's rules consider the screening effect of the 2s and 2p electrons (0.35 each) and the 1s electrons (0.85 each), resulting in a new effective nuclear charge of 3.25.
What is the difference between the effective nuclear charge for a 4s and a 3d electron in vanadium according to Slater's rules?
-The effective nuclear charge for a 4s electron in vanadium is 3.3, while for a 3d electron it is higher at 4.3, indicating that the 3d electron is more attracted to the nucleus.
Why is it typically easier to remove a 4s electron than a 3d electron in transition metals?
-It is easier to remove a 4s electron because it has a lower effective nuclear charge compared to a 3d electron, as shown by the calculations using Slater's rules.
Outlines
📚 Introduction to Slater's Rules
This paragraph introduces the topic of Slater's Rules, which are used to refine the approximation of the screening or shielding constant in calculating the effective nuclear charge. It acknowledges that most general chemistry students won't need to know these rules, but some, particularly those in majors classes, might. The speaker, Chad, welcomes viewers to his science learning channel and mentions his courses for various exams, highlighting a new general chemistry playlist. The paragraph ends with an encouragement to subscribe for updates.
🔍 Understanding Slater's Rules with Examples
The paragraph delves into Slater's Rules by working through examples to illustrate how they provide a better approximation of the effective nuclear charge. It corrects previous assumptions about electron screening within the same shell and between core electrons, introducing specific screening values. The examples start with helium, explaining how to calculate the new screening constant and effective nuclear charge, revealing a lower attraction of the valence electron to the nucleus than previously thought.
🔬 Applying Slater's Rules to Larger Atoms
This section continues the application of Slater's Rules with more complex examples, such as carbon and vanadium. It explains the process of grouping electrons in the same shell and calculating the screening constant for an electron in a different shell. The paragraph provides a step-by-step calculation for carbon, revealing a lower effective nuclear charge than expected, which impacts the understanding of atomic radius. It also addresses the challenge of applying Slater's Rules to transition metals like vanadium, emphasizing the importance of ordering electron configurations correctly.
📘 Conclusion and Additional Resources
The final paragraph wraps up the discussion on Slater's Rules, comparing the effective nuclear charges calculated for 4s and 3d electrons in vanadium. It explains why the 3d electron is more attracted to the nucleus, aligning with the tendency to remove 4s electrons first. The speaker invites students who found the lesson helpful to engage with the content and offers additional resources, such as quizzes and practice exams, through a master course with a free trial link provided in the description.
Mindmap
Keywords
💡Slater's Rules
💡Effective Nuclear Charge
💡Screening/Shielding Constant
💡General Chemistry
💡Valence Electrons
💡Atomic Radius
💡Electron Configuration
💡1s Electrons
💡Transition Metals
💡Noble Gases
Highlights
Slater's rules are used to approximate the screening or shielding constant for effective nuclear charge calculation.
Most general chemistry students won't need to know Slater's rules, but some may for advanced classes.
Slater's rules provide a better approximation of effective nuclear charge than previous lessons.
Effective nuclear charge influences atomic radius and was previously approximated based on valence electrons.
Slater accounted for the fact that electrons in the same shell do screen each other, contrary to previous assumptions.
Core electrons have a screening value less than one, differing from the previous assumption of one.
Examples are used to demonstrate the application of Slater's rules, starting with helium.
For helium, the effective nuclear charge is calculated to be 1.7, not the previous approximation of 2.
Carbon is used as an example to show the calculation of effective nuclear charge with Slater's rules.
The effective nuclear charge for carbon is found to be 3.25, a more precise value than the previous approximation of 4.
Vanadium is used to demonstrate the application of Slater's rules to transition metals.
Effective nuclear charge for vanadium's 4s electron is calculated to be 3.3.
Effective nuclear charge for vanadium's 3d electron is higher at 4.3, indicating a stronger attraction to the nucleus.
The difference in effective nuclear charge explains why a 4s electron is removed before a 3d electron in vanadium.
Slater's rules are significant for a subset of chemistry students who need a more precise understanding of atomic structure.
The instructor offers additional resources for practice and mastery of general chemistry concepts.
Transcripts
Browse More Related Video

Using Slater's Rules: 3 Examples

How To Use Slater's Rule to Estimate The Effective Nuclear Charge

Trick for Slater's Rule, calculation of screening constant and effective nuclear charge.
Effective Nuclear Charge, Shielding effect, & Periodic Properties Tutorial; Crash Chemistry Academy
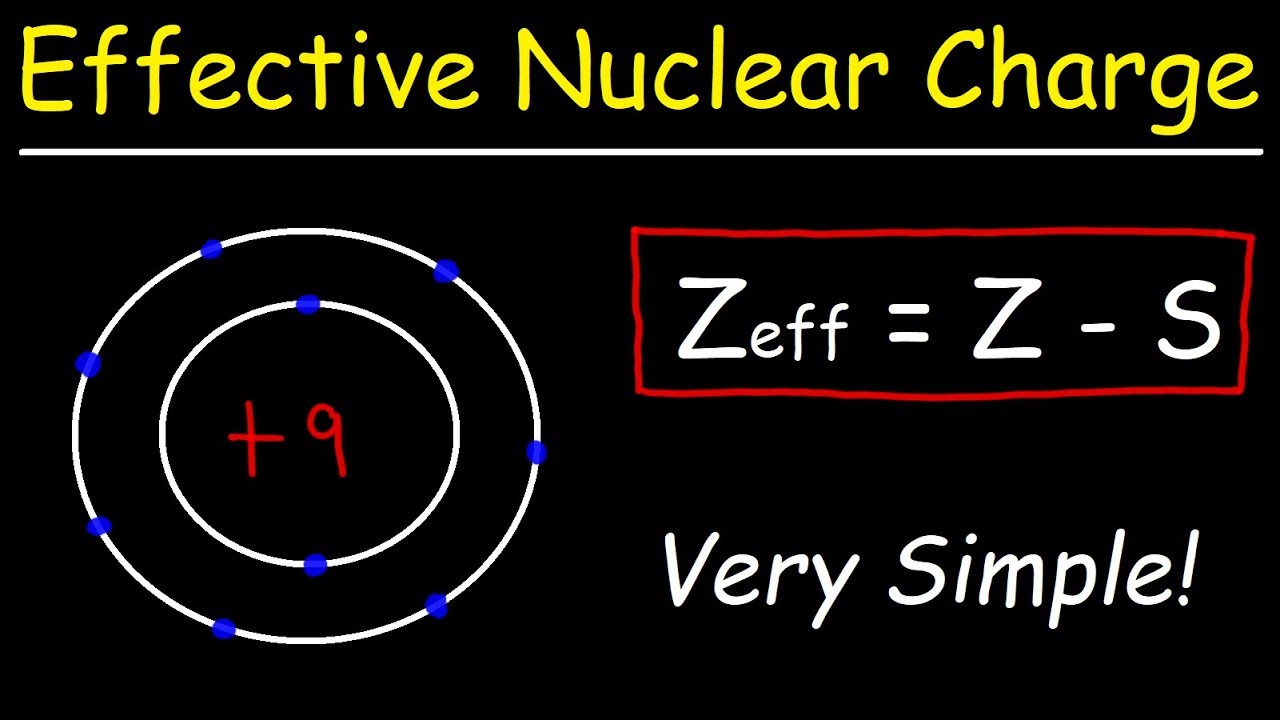
How To Calculate The Effective Nuclear Charge of an Electron

7.5 Periodic Trends | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: