How to Find the Charge of an Ion! (The Octet Rule)
TLDRThis educational video script explains the concept of ionic charges using the octet rule, focusing on how atoms strive to achieve a full valence shell. It illustrates the process with nitrogen gaining electrons to become an anion and sodium losing an electron to become a cation. The script also introduces general trends for elements based on their valence electrons, helping to predict ion formation and charges, ultimately unlocking a deeper understanding of chemistry for students.
Takeaways
- 🚀 The video explains how to determine the charge of an ion, focusing on the principles of the octet rule.
- 🔬 A neutral atom has the same number of electrons as protons, which is equal to its atomic number.
- 🌀 The first shell of an atom can hold up to two electrons, while the second shell can hold up to eight electrons.
- 📚 The valence shell is the last shell with electrons, and valence electrons are those in the valence shell.
- 💭 Atoms have a 'lifelong dream' to fill their valence shell completely, which drives their chemical behavior.
- ➕ When an atom gains electrons to fill its valence shell, it becomes negatively charged (an anion).
- ➖ Conversely, when an atom loses electrons to achieve a full valence shell, it becomes positively charged (a cation).
- 📉 Elements with 1-3 valence electrons tend to lose electrons, while those with 5-7 valence electrons tend to gain electrons.
- 📈 Noble gases with 8 valence electrons (except helium with 2) are stable and typically do not form ions.
- 🔄 The periodic table can be categorized by valence electrons, which helps predict an element's ion formation tendency.
- 📝 The overall charge of an ion is calculated by adding the charge of protons to the charge of electrons.
- 📚 The script provides step-by-step examples using nitrogen, sodium, calcium, and arsenic to illustrate ion charge determination.
Q & A
What is the fundamental principle used to find the charge of an ion?
-The fundamental principle used to find the charge of an ion is based on the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full valence shell, typically with eight electrons.
How many electrons does a neutral atom of nitrogen have?
-A neutral atom of nitrogen has seven electrons, which is equal to its atomic number.
What is the valence shell in the context of the nitrogen atom?
-The valence shell is the outermost shell that contains electrons. For nitrogen, the second shell is its valence shell.
What are valence electrons and how many does nitrogen have in its valence shell?
-Valence electrons are the electrons in the valence shell. Nitrogen has five valence electrons in its second shell when it is neutral.
What is the 'lifelong dream' of every atom as described in the script?
-The 'lifelong dream' of every atom is to have a completely full valence shell, which typically means having eight electrons in the outermost shell.
How does nitrogen achieve its 'lifelong dream' according to the script?
-Nitrogen achieves its 'lifelong dream' by gaining three more electrons to completely fill its second shell, resulting in eight electrons in the valence shell.
What is the ion charge of nitrogen when it gains three electrons?
-When nitrogen gains three electrons, its ion charge becomes negative three, written as N^3-.
What is the atomic number of sodium and how does it relate to the number of electrons in a neutral atom?
-The atomic number of sodium is 11, which means a neutral atom of sodium has 11 electrons.
Why does sodium tend to lose an electron rather than gain seven to fill its valence shell?
-Sodium tends to lose an electron because it is the same amount of work to gain or lose an electron, and losing one electron is less work than gaining seven.
What is the ion charge of sodium after it loses one electron?
-After losing one electron, sodium has an ion charge of positive one, written as Na^+.
What are the general trends for elements with different numbers of valence electrons when forming ions?
-Elements with one to three valence electrons tend to lose electrons to achieve a full valence shell, becoming cations. Elements with five to seven valence electrons tend to gain electrons to reach eight, becoming anions. Elements with four valence electrons can either gain or lose electrons, and elements with eight valence electrons typically remain neutral.
How can you predict the ion charge of elements in the first column of the periodic table?
-Elements in the first column of the periodic table, which have one valence electron, will typically form ions with a charge of plus one.
What is the ion charge for elements in the second column of the periodic table?
-Elements in the second column, with two valence electrons, will form ions with a charge of plus two.
How does the script describe the behavior of elements with four valence electrons when forming ions?
-Elements with four valence electrons are on the edge and can either gain or lose four electrons depending on the circumstances, resulting in either a positive or negative ion.
What is the ion charge for elements with eight valence electrons?
-Elements with eight valence electrons, such as the noble gases, typically do not form ions as they are already satisfied with a full valence shell, and their ion charge remains zero.
Outlines
🔬 Understanding Ions and the Octet Rule
This paragraph introduces the concept of finding the charge of an ion using the principles of the octet rule. It begins with nitrogen as an example, explaining that a neutral atom has an equal number of protons and electrons, which corresponds to its atomic number. The script details the electron configuration of nitrogen, emphasizing the importance of valence electrons and the desire of atoms to achieve a full valence shell. It humorously personifies atoms with the 'dream' of a full valence shell and illustrates how nitrogen would achieve this by gaining three electrons, resulting in a negatively charged ion. The process of calculating the ion charge by adding the charges of protons and electrons is explained, leading to the conclusion that the nitrogen ion would have a charge of negative three.
📚 Ion Formation in Sodium and the Role of Valence Electrons
The second paragraph explores ion formation using sodium as an example. It describes the electron configuration of a neutral sodium atom, which has 11 electrons corresponding to its atomic number. The script introduces additional rules regarding an atom's 'lifelong dream' of a full valence shell and the ease of losing an electron for atoms like sodium, which has its outermost shell not fully occupied. It explains that sodium would rather lose one electron than gain seven to achieve a full shell, thus becoming a positively charged ion. The calculation of the ion charge for sodium is detailed, showing that after losing an electron, it becomes a sodium ion with a charge of positive one, or Na⁺. The concept of cations and anions is also introduced, with sodium's positive ion being a cation.
🌐 Periodic Trends in Ion Formation and Valence Electrons
This paragraph discusses the overall trends in ion formation based on the number of valence electrons and their position in the periodic table. It categorizes elements into groups based on their valence electrons and predicts their ion formation tendencies. Elements with one to three valence electrons, found on the left side of the periodic table, tend to lose electrons to achieve a full outer shell, forming positive ions (cations). Conversely, elements with five to seven valence electrons, closer to achieving a full shell, tend to gain electrons, resulting in negative ions (anions). Elements with four valence electrons can either gain or lose electrons depending on the circumstances, while those with a full octet of eight valence electrons remain neutral and do not form ions. The script also provides a brief overview of the charges typically formed by elements in different columns of the periodic table.
📝 Practice Problems: Predicting Ions for Calcium and Arsenic
The final paragraph presents practice problems to predict the ions for calcium and arsenic, applying the concepts learned in the previous sections. Calcium, with two valence electrons, is predicted to lose electrons and form a calcium ion with a charge of +2 (Ca²⁺), as it follows the trend of elements in the second column of the periodic table. Arsenic, with five valence electrons, is expected to gain electrons to achieve a full octet, resulting in an arsenic ion with a charge of -3 (As³⁻). The process involves calculating the ion charge by considering the number of protons and the change in the number of electrons. The paragraph reinforces the idea that understanding valence electrons and their behavior in ion formation is crucial for solving chemistry problems and provides a comprehensive approach to predicting ion charges.
Mindmap
Keywords
💡Octet Rule
💡Atomic Number
💡Valence Electrons
💡Ion
💡Electron Configuration
💡Neutral Atom
💡Ion Charge
💡Anion
💡Cation
💡Periodic Table
💡Noble Gases
Highlights
Introduction to the concept of finding the charge of an ion using the octet rule.
Explanation of how a neutral atom has the same number of electrons as protons, equal to the atomic number.
Demonstration of how to draw electrons around a nitrogen nucleus to determine its electron configuration.
Description of the first shell holding up to two electrons and the second shell holding up to eight.
Definition of the valence shell and valence electrons in relation to the last shell with electrons.
The idea that atoms have a lifelong dream to have a completely full valence shell.
Example of nitrogen gaining three electrons to fulfill its dream of a full valence shell.
Calculation of the overall charge of an atom by adding the charge of protons to the charge of electrons.
Illustration of how nitrogen's charge changes from neutral to negative three after gaining electrons.
Introduction of sodium as an example and its electron configuration with atomic number 11.
Explanation of sodium's preference to lose an electron rather than gain seven due to the same amount of work for both processes.
Calculation of sodium's ion charge after losing an electron, resulting in a positive one charge.
General trends of ion formation based on the number of valence electrons in the periodic table.
Prediction of ion charges for elements with one to three valence electrons, tending to lose electrons and become cations.
Prediction of ion charges for elements with five to seven valence electrons, tending to gain electrons and become anions.
Explanation of elements with four valence electrons being on the edge, potentially gaining or losing electrons.
Practice problems predicting the ions for calcium and arsenic using the established rules and trends.
Summary of the periodic table trends showing how elements form ions with specific charges based on their valence electron count.
Encouragement for students to practice and understand the concepts to unlock more chemistry problems.
Transcripts
Browse More Related Video

What are Ions?

Noble gas configuration (old, low volume)

GCSE Chemistry - What is Ionic Bonding? How Does Ionic Bonding Work? Ionic Bonds Explained #14

Noble gas configuration | Electronic structure of atoms | Chemistry | Khan Academy
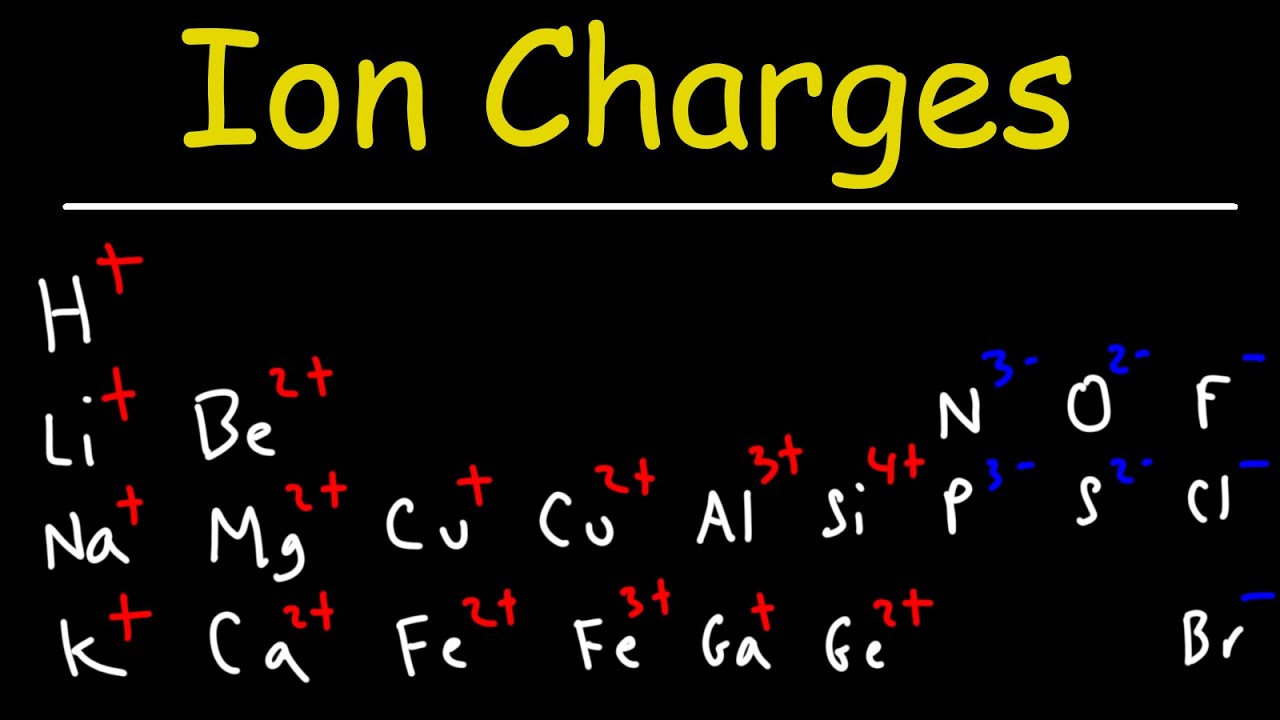
How To Determine The Charge of Elements and Ions - Chemistry

Introduction to Oxidation States
5.0 / 5 (0 votes)
Thanks for rating: