Energy, Ionic Solids, Metals, & Alloys - AP Chem Unit 2, Topics 2-4
TLDRIn this engaging AP Chemistry lesson, Jeremy Krug delves into the intricacies of chemical bonding, focusing on the energy dynamics between atoms. He explains the concept of bond length and bond enthalpy using a graph to illustrate potential energy changes as atoms approach each other. Krug then explores ionic bonding, detailing the electron transfer between metals and non-metals, and how this results in the formation of cations and anions. He emphasizes the strength of ionic bonds due to electrostatic attractions and discusses how the bond's charge magnitude and ionic size influence the melting points of compounds. The instructor also touches on the properties of ionic compounds, their solubility, and their behavior as electrolytes. Moving on to metallic bonding, Krug describes the delocalized electrons in metals, which enable electrical conductivity, and distinguishes between substitutional and interstitial alloys, using steel as an example of the latter. This comprehensive overview not only educates but also piques the interest of students in the fundamental principles governing chemical bonds and material properties.
Takeaways
- 📈 The bond length between two atoms is the distance at which the potential energy is at its lowest, in this case, 200 picometers.
- ⚡ The bond enthalpy or bond energy is the energy at the lowest point of the potential energy graph, which represents the energy released during bond formation, here approximately 250 kilojoules per mole.
- ⚖️ Positive and negative charges attract each other due to electrostatic attractions, which are very strong in ionic bonds.
- 🔥 Ionic bonds are generally strong and are formed between a metal and a non-metal, leading to the formation of cations and anions.
- 🧊 The melting point of ionic compounds increases with the magnitude of charge; higher charges result in stronger electrostatic attractions and higher melting points.
- 📊 When comparing ionic compounds with the same charge, the size of the ions becomes the determining factor for melting points; larger ions result in weaker forces and lower melting points.
- 🔩 Metallic bonding involves delocalized electrons, which allows metals to conduct electricity as the electrons can move freely within the metal structure.
- 🤝 In metallic alloys, substitutional alloys involve atoms of different elements substituting into the main element's lattice, while interstitial alloys involve smaller atoms filling the spaces between the main atoms.
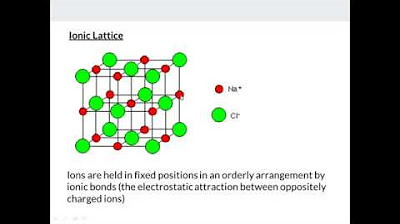
- 💎 Ionic compounds are typically brittle, have high melting points, are soluble in polar solvents like water, and have an orderly crystal lattice structure.
- 🧲 Dissolved ionic compounds conduct electricity because they form electrolytes with charged particles that move freely in solution.
- 🔑 Predicting the relative melting points of ionic compounds involves looking at the charge differential and the size of the ions; larger charges and smaller ions result in higher melting points.
Q & A
What is the significance of the bond length in the context of the graph discussed in the script?
-The bond length is the distance between two atoms where the potential energy between them is at its lowest. In the script, this occurs at an internuclear distance of 200 picometers.
What is the bond enthalpy or bond energy between two atoms, and how is it represented on the graph?
-The bond enthalpy, or bond energy, is the energy at the point where the potential energy between two atoms is at its lowest. It is represented as a negative number on the graph, indicating the energy released during the formation of a chemical bond. In the example given, it is about 250 kilojoules per mole.
Why are chemical bonds forming an exothermic process?
-Chemical bonds forming are exothermic because they release energy. This is due to the fact that atoms have lower potential energy when they are closer together and form a bond, as opposed to when they are farther apart.
How does the bond enthalpy relate to the energy required to break bonds?
-Bond enthalpy refers to the amount of energy required to break the bonds. It is always a positive number because it represents the energy input needed to separate the bonded atoms.
What is ionic bonding and how does it differ from covalent bonding?
-Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, typically formed between a metal and a non-metal. It differs from covalent bonding, which involves the sharing of electrons between atoms.
What happens to the electron configurations of sodium and chlorine when they form an ionic bond?
-When sodium and chlorine form an ionic bond, sodium loses its one valence electron (3s1) to become a positively charged cation, achieving a stable electron configuration. Chlorine gains this electron to complete its octet, becoming a negatively charged anion.
Why are ionic bonds strong and what is the force responsible for this strength?
-Ionic bonds are strong due to the electrostatic attractions between the positively and negatively charged ions. These attractions are a result of the opposite charges attracting each other, leading to a very stable bond.
How does the melting point of an ionic compound relate to the magnitude of the charges of the ions involved?
-The melting point of an ionic compound increases with the magnitude of the charges of the ions. This is because a higher charge differential leads to stronger electrostatic attractions, requiring more energy to break the bonds and thus resulting in a higher melting point.
What is Coulomb's law and how does it apply to predicting the melting points of ionic compounds?
-Coulomb's law states that the force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. It applies to predicting the melting points of ionic compounds by considering the charge magnitude and the distance between ions, with larger charges and smaller distances leading to stronger forces and higher melting points.
How does the size of the ions influence the melting point of ionic compounds with the same charge?
-For ionic compounds with the same charge, the size of the ions becomes the determining factor for the melting point. Larger ions result in weaker forces due to increased distance between the charges, leading to a lower melting point. Conversely, smaller ions have stronger attractions and thus a higher melting point.
What is the lattice energy and how does it relate to the strength of the ionic compound?
-Lattice energy is the energy released when ions are combined into an ionic compound. The stronger the electrostatic forces between the ions, the more energy is released, indicating a stronger ionic compound.
What are the properties of ionic compounds and how do they differ from metallic compounds?
-Ionic compounds have high melting points, are brittle, usually conduct electricity when dissolved in water, and are soluble in polar solvents. They have a crystal lattice structure held together by electrostatic forces. Metallic compounds, on the other hand, have delocalized electrons that allow them to conduct electricity and heat. They are malleable and ductile, and do not form a crystal lattice structure.
Outlines
🔬 Understanding Atomic Energy and Bonding
Jeremy Krug introduces the concept of energy between two atoms, focusing on ionic and metallic bonding. He explains the potential energy graph, identifying bond length at the lowest point of energy (200 picometers) and bond enthalpy (-250 kilojoules per mole). The video also covers how sodium and chlorine form an ionic bond, resulting in oppositely charged ions that attract due to electrostatic forces, leading to strong ionic bonds. The strength of these bonds is illustrated by the high melting points of compounds like sodium fluoride and magnesium fluoride.
📈 Predicting Melting Points of Ionic Compounds
The paragraph discusses the relationship between ionic charges and melting points of ionic compounds. It explains that as the magnitude of charge increases, so does the melting point, following Coulomb's law. The video also explores the impact of ionic size on melting points, noting that larger ions result in weaker forces and lower melting points. The concept of lattice energy is introduced, which is the energy released when ions form an ionic compound. The video concludes with a method to predict the relative melting points of ionic compounds by first examining the charges and then the ionic size if the charges are the same.
🌟 Properties of Ionic Compounds
This section delves into the properties of ionic compounds, such as their high melting points, brittleness, and ability to conduct electricity when dissolved in water, classifying them as electrolytes. It also touches on their solubility in polar solvents like water. Ionic compounds are characterized by an orderly, repeating crystal structure known as a crystal lattice, which is maintained by electrostatic forces. The video emphasizes that ionic compounds do not exist as individual molecules but as a continuous three-dimensional lattice.
🛠️ Metallic Bonds and Alloys
The video explains the nature of metallic bonding, where electrons are delocalized and can move freely, allowing metals and alloys to conduct electricity. It differentiates between two types of alloys: substitutional alloys, where different elements substitute into the lattice, and interstitial alloys, where smaller atoms like carbon in steel occupy spaces between atoms, acting as a hardening agent. The video also notes that different types of steel have varying carbon and element content, leading to diverse properties.
📚 Summary and Upcoming Content
Jeremy Krug concludes the video by summarizing the content covered in sections 2.2, 2.3, and 2.4, which include atomic energy, ionic and metallic bonding, and the properties of ionic compounds and alloys. He encourages viewers to like the video if they found it informative and teases the next video in the series, which will cover unit 2, section 5.
Mindmap
Keywords
💡Potential Energy
💡Bond Length
💡Bond Enthalpy
💡Ionic Bonding
💡Electrostatic Attractions
💡Melting Point
💡Coulomb's Law
💡Lattice Energy
💡Ionic Compounds
💡Metallic Bonding
💡Substitutional Alloys
💡Interstitial Alloys
Highlights
The potential energy between two atoms is lowest at the bond length, which is identified as the point where the graph of potential energy versus distance hits its lowest point.
The bond enthalpy or bond energy is the energy at the lowest point of the potential energy graph, representing the energy released when the atoms form a chemical bond.
Chemical bond formation is an exothermic process, hence the bond enthalpy is represented by a negative number, while the energy required to break the bond is positive.
Ionic bonding involves a metal and a non-metal, where the metal donates an electron to the non-metal, resulting in the formation of oppositely charged ions attracted to each other by electrostatic forces.
The strength of ionic bonds can be inferred from their high melting points, with compounds like sodium fluoride, magnesium fluoride, and aluminum fluoride having melting points over 1000°C.
Coulomb's law explains the relationship between the magnitude of charge and the melting point of ionic compounds, where a higher charge differential results in a higher melting point.
When the charges of ions are the same, the size of the ions becomes the determining factor for the melting point, with smaller ions leading to stronger attractions and higher melting points.
Lattice energy is the energy released when ions combine to form an ionic compound, with stronger forces resulting in more energy release.
Ionic compounds have a high melting point, are brittle, usually dissolve in water to conduct electricity, and have a well-ordered crystal lattice structure.
Metals have delocalized electrons, allowing them to conduct electricity and heat, which is likened to a 'sea of electrons' around positively charged nuclei.
Substitutional alloys, such as brass, bronze, and pewter, involve different elements substituting into the lattice of the main element.
Interstitial alloys, like steel, involve smaller atoms, such as carbon in steel, occupying the spaces between the main atoms, acting as a hardening agent.
Different types of steel are made by varying the amounts of carbon and other elements, resulting in different properties.
Ionic compounds do not exist as individual molecules but rather as a three-dimensional crystal lattice held together by electrostatic forces.
The relative sizes of ions can be used to predict the relative melting points of ionic compounds, with larger ions resulting in weaker forces and lower melting points.
Metallic bonding is characterized by the delocalization of electrons, which allows for the flow of electricity and the formation of alloys with unique properties.
The video provides a comprehensive understanding of the concepts of bond length, bond enthalpy, ionic and metallic bonding, and the properties of ionic compounds and alloys.
Transcripts
Browse More Related Video

AP Chem - Unit 2 Review - Molecular & Ionic Compound Structure and Properties

8.1 Ionic, Covalent, and Metallic Bonding | High School Chemistry

[H2 Chemistry] 2023 Topic 2 Chemical Bonding 1

Ionic Bonding [IB Chemistry SL/HL]

BTEC Applied Science: Unit 1 Chemistry Ionic Bonding
Chemical Bonding Concepts (Part 2)
5.0 / 5 (0 votes)
Thanks for rating: