24.1 Classification of Monosaccharides | Organic Chemistry
TLDRThe video script presents an in-depth exploration of monosaccharides, the fundamental building blocks of carbohydrates. It begins with the basic classification of carbohydrates based on their chemical structure, highlighting the typical formula Cn(H2O)n, and introduces the concept of Fischer projections commonly used in sugar chemistry. The lesson delves into the structure of monosaccharides, distinguishing between aldoses and ketoses based on the presence of an aldehyde or ketone group. The importance of stereochemistry is emphasized, explaining the D and L configurations for determining the chirality of sugars. The script also covers the concept of enantiomers, diastereomers, and epimers, which are crucial for understanding the different stereoisomers of monosaccharides. Furthermore, the lecture discusses the cyclization of monosaccharides to form cyclic hemiacetals, focusing on the stability of five and six-membered rings, known as furanoses and pyranoses, respectively. The distinction between alpha and beta anomers is clarified, illustrating the impact of the hydroxyl group's position on the cyclic form. The script concludes with a teaser for the next lesson, which will focus on the reactions of monosaccharides, inviting viewers to engage with the content through likes and comments to support the dissemination of the educational material.
Takeaways
- 🍬 Monosaccharides are the simplest form of carbohydrates, consisting of a single sugar molecule.
- 🔍 Monosaccharides follow a general formula of Cn(H2O)n, indicating the presence of carbon, hydrogen, and oxygen.
- 🧬 Monosaccharides can be classified as aldoses (with an aldehyde group) or ketoses (with a ketone group).
- 📏 The number of carbon atoms in a monosaccharide determines its specific name, such as triose, tetrose, pentose, hexose, and so on.
- 🔣 Fischer projections are commonly used to represent the structure of monosaccharides, with horizontal bonds as wedge bonds and vertical bonds as dashed bonds.
- ⛔ Monosaccharides can have multiple chiral centers, leading to various stereoisomers, which are important for understanding their three-dimensional shape.
- 🔄 D and L configurations in sugars refer to the arrangement of groups around the lowest chiral center (highest numbered) in a Fischer projection.
- 🤝 Enantiomers are non-superimposable mirror images of each other, while diastereomers are stereoisomers that are not mirror images but also not identical.
- 🔑 Epimers are a special type of diastereomers that differ at only one chiral center.
- ♻️ Monosaccharides can exist in both open-chain and cyclic forms, with cyclic forms being more stable, especially for aldoses.
- 🔁 Mutarotation is the process by which pure alpha or beta anomers equilibrate to form a mixture of both when dissolved in water.
Q & A
What is the basic formula that carbohydrates follow?
-Carbohydrates follow the general formula Cn(H2O)n, where for every carbon atom, there is an equivalent of a water molecule in the structure.
What are the two functional groups that monosaccharides can have?
-Monosaccharides can have either an aldehyde or a ketone functional group.
How are aldehyde sugars and ketone sugars classified in terms of their suffixes?
-Aldehyde sugars are called aldoses and end with an '-ose' suffix, while ketone sugars are called ketoses.
What is the significance of Fischer projections in sugar chemistry?
-Fischer projections are commonly used in sugar chemistry to represent the stereochemistry of sugars, with horizontal bonds as wedge bonds and vertical bonds around a chiral center as dashed bonds.
How are monosaccharides classified based on the number of carbon atoms they contain?
-Monosaccharides are classified as trioses (3 carbons), tetroses (4 carbons), pentoses (5 carbons), hexoses (6 carbons), and heptoses (7 carbons).
What are the D and L configurations in relation to monosaccharides?
-The D and L configurations refer to the chirality of the lowest (or highest numbered) chiral center in a Fischer projection of a sugar. If the hydroxyl group is on the right for the highest numbered chiral center, it is a D sugar, and if it is on the left, it is an L sugar.
What are enantiomers and how do they relate to sugars?
-Enantiomers are non-superimposable mirror images of each other. In relation to sugars, D and L forms of a sugar are enantiomers, and they differ by the inversion of all chiral centers.
What is the difference between diastereomers and epimers?
-Diastereomers are stereoisomers that are not mirror images and not identical, while epimers are a special type of diastereomers that differ at only one chiral center.
How do monosaccharides form cyclic structures?
-Monosaccharides can cyclize in solution to form cyclic hemiacetals, which can be either six-membered rings called pyranoses or five-membered rings called furanoses.
What is the term used to describe the carbonyl carbon that becomes a chiral center in the cyclic hemiacetal form of a sugar?
-The carbonyl carbon that becomes a chiral center in the cyclic hemiacetal form is called the anomeric carbon.
What are the two terms used to describe the configuration at the anomeric carbon in cyclic forms of sugars?
-The two terms used to describe the configuration at the anomeric carbon are Alpha and Beta, which refer to the relative positions of the hydroxyl group and the CH2OH group in relation to the ring.
What is mutarotation and why is it significant in the context of sugars?
-Mutarotation is the process of equilibration between the open chain form and the two different cyclic forms (alpha and beta anomers) of a sugar when it is in solution. It is significant because it means that a pure anomer of a sugar will not remain pure once in solution due to the establishment of an equilibrium.
Outlines
📚 Introduction to Monosaccharides and Carbohydrates
The video begins with an introduction to monosaccharides, which are the focus of the first lesson in a chapter on carbohydrates. The instructor explains that they were not originally part of their organic chemistry curriculum due to the university's syllabus but are now covering them due to a student's request. Monosaccharides are the simplest form of carbohydrates, following a general formula where each carbon is equivalent to a water molecule. The video uses Fischer projections to represent the structures of monosaccharides, highlighting the difference between horizontal (wedge) and vertical (dashed) bonds at chiral centers. The lesson also distinguishes between aldoses and ketoses based on the presence of an aldehyde or ketone group, respectively.
🔍 D and L Configurations in Monosaccharides
The second paragraph delves into the D and L configurations of monosaccharides, explaining that these terms originally referred to the direction of plane-polarized light rotation but are now used to describe the chirality of the lowest or highest numbered chiral center in a Fischer projection. The video clarifies that D and L sugars are enantiomers, requiring the inversion of all chiral centers to interconvert. Additionally, the concept of diastereomers is introduced, which are stereoisomers that are not mirror images, and epimers, a special type of diastereomers differing at a single chiral center.
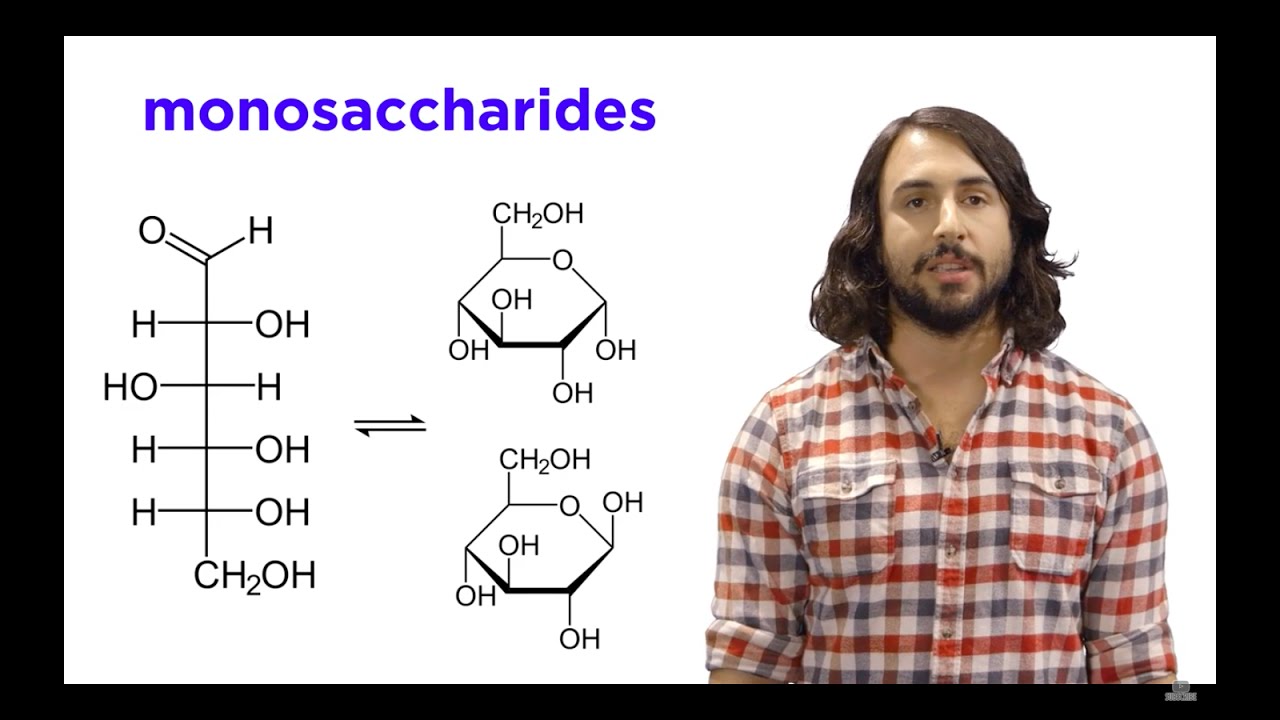
🔄 Cyclization of Monosaccharides into Hemiacetals
The third paragraph discusses the cyclization of monosaccharides into cyclic hemiacetals, which are more stable than their linear forms. The video explains how monosaccharides can form either five-membered (furanose) or six-membered (pyranose) rings. It details the process of forming cyclic hemiacetals through the nucleophilic attack of an alcohol group on an aldehyde, resulting in the formation of a new bond between the oxygen and the terminal carbon. The video also introduces the concept of Haworth projections for representing cyclic forms and explains the orientation of hydroxyl groups in these projections.
🔑 Anomeric Carbon and the Alpha/Beta Designation
The fourth paragraph focuses on the concept of the anomeric carbon, which is the carbon involved in the formation of a new chiral center upon cyclization. It explains that this carbon can lead to two different forms known as anomers, distinguished as Alpha or Beta based on the orientation of the hydroxyl group and the ch2oh group relative to each other. The video also discusses the common occurrence of D sugars in living organisms and provides a mnemonic for determining the Alpha or Beta configuration, particularly for D sugars.
🌀 Equilibrium of Open Chain and Cyclic Forms
The fifth paragraph explores the equilibrium between the open chain form and the cyclic forms of monosaccharides, specifically focusing on the pyranose ring. It explains that while pure anomers can be isolated, they will undergo an equilibration process called mutarotation when placed in solution, resulting in a mixture of both anomers. The video also touches on the formation of five-membered rings in fructose and how these biologically relevant forms are more common within cells despite six-membered rings being more stable.
🔬 Recognizing Anomeric Carbon and Hemiacetals
The final paragraph provides a wrap-up on recognizing the anomeric carbon in hemiacetals by identifying the carbon bonded to two oxygens, one being part of the ring. It summarizes the lesson on the classification of monosaccharides and teases the next lesson, which will cover the reactions of monosaccharides. The video also encourages viewers to like and comment to help the content reach a broader audience and promotes the instructor's organic chemistry Master course and a course designed for the ACs standardized final.
Mindmap
Keywords
💡Monosaccharides
💡Carbohydrate Formula
💡Fischer Projection
💡Aldehydes and Ketones
💡Chiral Centers
💡D and L Configuration
💡Diastereomers
💡Cyclic Hemiacetals
💡Anomers
💡Mutarotation
💡Epimers
Highlights
Carbohydrates are classified based on their typical formula of Cn(H2O)n, where 'n' represents the number of carbons.
Monosaccharides are the simplest form of carbohydrates and can be either aldoses (with an aldehyde group) or ketoses (with a ketone group).
Fischer projections are commonly used in sugar chemistry to represent the structure of monosaccharides.
Every carbon in a monosaccharide that is not part of the carbonyl group typically has a hydroxyl group, often leading to multiple chiral centers.
Monosaccharides can be classified further based on the number of carbons they contain, such as trioses, tetroses, pentoses, hexoses, and so on.
The D and L designation for sugars refers to the configuration at the lowest or highest numbered chiral center in a Fischer projection.
Enantiomers are non-superimposable mirror images, while diastereomers are stereoisomers that are not mirror images.
Epimers are a special type of diastereomers that differ at only one chiral center.
Monosaccharides can cyclize to form cyclic hemiacetals, with five-membered rings called furanoses and six-membered rings called pyranoses.
The anomeric carbon is the carbon involved in the formation of the cyclic hemiacetal, which can lead to two different forms: alpha and beta.
The alpha and beta anomers interconvert in solution through a process called mutarotation.
Fructose, a ketose, commonly forms a five-membered ring, which is the most biologically relevant form.
The cyclic forms of monosaccharides are more stable than their open-chain forms, especially in living systems.
The orientation of the hydroxyl group and the ch2oh group on the anomeric carbon determines whether the form is alpha or beta.
D-sugars are more common in living organisms compared to L-sugars, with D-sugars predominantly found in nature.
The cyclic forms of sugars can be represented in different conformations, such as Haworth projection or chair conformation.
The equilibration between the open chain and cyclic forms of sugars in solution is an important concept in understanding their stability and reactivity.
The video provides a comprehensive overview of monosaccharide classification, which is crucial for understanding more complex carbohydrates.
Transcripts
Browse More Related Video
Carbohydrates Part 1: Simple Sugars and Fischer Projections

FC8 Unit 4 AOS2 Simple carbohydrates

12. Carbohydrates/Introduction to Membranes

24.2 Reactions of Monosaccharides | Organic Chemistry

24.3 Disaccharides and Polysaccharides | Organic Chemistry

Biochemical Building Blocks & Fischer and Haworth Projections: Crash Course Organic Chemistry #48
5.0 / 5 (0 votes)
Thanks for rating: