10. Radioactive Decay Continued
TLDRThe transcript is a detailed lecture on nuclear science and engineering, focusing on radioactive decay, nuclear activation analysis (NAA), and the mathematical modeling of decay chains. The lecturer, Michael Short, addresses the misconception that the field is purely computational, emphasizing the importance of understanding both theory and practical lab work. He introduces the concept of decay constant and half-life, explaining how these factors determine the rate of decay for radioactive isotopes. Short also discusses various decay mechanisms, including alpha and beta decay, positron emission, and electron capture. A significant portion of the lecture is dedicated to the practical application of NAA, where students are asked to bring samples for analysis. The lecture concludes with an exploration of the mathematics behind decay chains, guiding students through the process of setting up differential equations to model serial radioactive decay, and the impact of neutron flux on isotope production and destruction in a reactor. The content is rich with educational insights and practical applications, providing students with a comprehensive understanding of nuclear reactions and their dynamics.
Takeaways
- 📚 The lecture discusses the misconception that nuclear science engineering is purely computational, emphasizing the importance of both theory and practical experiments.
- 🚀 MIT OpenCourseWare offers high-quality educational resources for free, supported by donations, and provides access to a wealth of materials from various MIT courses.
- 🤔 Student concerns about the lack of experiments in the course are addressed, highlighting the upcoming practical components, including work with the Nuclear Reactor Lab.
- 🧪 An assignment is introduced where students will perform Nuclear Activation Analysis (NAA) on samples they bring in, applying their knowledge of radioactive decay to determine impurities.
- 📈 The use of neutron activation analysis is explained, noting its high sensitivity for detecting impurities in materials, and how it will be utilized in the course for practical learning.
- 📉 The concept of decay constants and half-life is explored, showing how they are used to predict the behavior of radioactive substances over time.
- 🔬 The competitive mechanisms of radioactive decay, such as positron emission and electron capture, are discussed, along with their implications for the field of nuclear engineering.
- 🧬 The practical aspects of handling radioactive materials are covered, including safety considerations like avoiding fissionable and overly salty samples.
- 📊 The mathematical modeling of nuclear reactions, including the creation and destruction of isotopes, is introduced using differential equations.
- 🌟 The role of the Nuclear Reactor Lab and the use of reactors to manipulate power levels and understand the practical aspects of nuclear energy are highlighted.
- ⚙️ The lecture touches on the use of various nuclear techniques in real-world applications, such as environmental studies and the analysis of materials for unexpected elements.
Q & A
What is the main concern raised in the beginning of the transcript regarding nuclear science engineering?
-The main concern raised is whether everything in the field of nuclear science engineering is computational in nature, as the course so far has mostly focused on theory and simulations without conducting any experiments.
What is the purpose of the lab activities that the students are going to participate in?
-The purpose of the lab activities is to provide students with hands-on experience and practical understanding of the concepts learned in class, such as radioactive decay and nuclear activation analysis.
What is Nuclear Activation Analysis (NAA) and how is it used in the course?
-Nuclear Activation Analysis (NAA) is a sensitive method used to measure impurities in materials by irradiating samples in a nuclear reactor and analyzing the resulting radioactive isotopes. In the course, students will use NAA to determine the elemental composition of various samples, applying their knowledge of radioactive decay and the Bateman equations.
What types of materials can students bring for the NAA lab assignment?
-Students can bring non-fissionable, non-salty materials that weigh about 50 milligrams and fit within a specified container. Examples include food items, pet claws, or clothing samples.
How do the terms 'rabbit' and 'pig' relate to nuclear science in the context of the transcript?
-In the context of the transcript, a 'rabbit' refers to a small capsule used to hold and transport samples for irradiation in the nuclear reactor, while a 'pig' is a heavy container made of lead used for shielding and storing irradiated materials.
What is the significance of the acronym 'CRUD' in the transcript?
-CRUD stands for Chock River Unidentified Deposits, which refers to the gunk that builds up on fuel rods in nuclear power plants. The term is used to illustrate the use of acronyms in the nuclear industry and the speaker's study of such deposits.
What is the role of Michael Ames in the course?
-Michael Ames works at the Nuclear Reactor Lab and is responsible for conducting nuclear experiments and running the NAA lab. He assists the students in understanding the practical aspects of nuclear activation analysis and related experiments.
What are the different mechanisms for positron emission and electron capture in the context of radioactive decay?
-Positron emission involves a nucleus emitting a positron and converting a proton into a neutron, while electron capture involves a nucleus capturing an electron, also converting a proton into a neutron. Both mechanisms result in the same daughter products, but they involve different radiation emissions that can be detected and measured.
What is the difference between isometric transition (or gamma decay) and internal conversion?
-Isometric transition, or gamma decay, involves a nucleus transitioning from an excited state to a lower energy state by emitting a gamma ray. Internal conversion, on the other hand, involves a gamma ray interacting with an electron in the atom, resulting in the ejection of the electron and the emission of an X-ray or Auger electron.
What is the significance of the K-shell and L-shell in the context of electron capture and gamma decay?
-The K-shell and L-shell represent different energy levels of electrons in an atom. In electron capture, a nucleus can capture an electron from either the K-shell or L-shell, resulting in different energy emissions. In gamma decay, the energy of the emitted gamma ray can lead to the ejection of an electron from the K-shell or L-shell, resulting in different types of X-rays or Auger electrons.
How does the concept of half-life relate to the decay constant in radioactive decay?
-The half-life of a radioactive isotope is the time it takes for half of the isotope's atoms to decay. The decay constant, lambda, is a measure of the decay rate of the isotope. The half-life is inversely related to the decay constant; a larger decay constant results in a shorter half-life, indicating a faster decay rate.
Outlines
📚 Introduction to Nuclear Science and Education at MIT
The paragraph introduces the video's context, emphasizing the importance of both theoretical knowledge and practical experiments in the field of nuclear science and engineering. It addresses a question about the computational nature of the field and assures that hands-on lab activities are included. The speaker, Michael Short, also introduces a guest, Mike Ames from the Nuclear Reactor Lab, who will assist with lab activities.
🧪 Nuclear Activation Analysis (NAA) and its Applications
This section delves into the specifics of Nuclear Activation Analysis (NAA), a highly sensitive method for measuring impurities in materials. The speakers discuss the practical aspects of conducting an NAA, including the use of a nuclear reactor and the requirement for students to bring in samples. They also touch upon the calculation of radioactive decay and the Bateman equations, which are essential for determining the impurities in the samples.
🚀 Engaging Students with Interactive Experiments
The paragraph discusses the importance of engaging students with interactive experiments. It outlines an assignment where students must bring in a sample to be analyzed for impurities. The assignment is not graded but is a requirement for the problem set. The dialogue also humorously explores various potential sample sources, such as food items, fingernails, or pet claws, emphasizing creativity and practicality in the selection of samples.
🔍 Detecting Elements Using NAA and Reactor Operations
The speakers provide a detailed overview of the elements that can be detected using NAA, including those with short half-lives. They discuss the process of irradiating samples in the reactor and the subsequent decay and counting procedures. The paragraph also covers the practical aspects of handling samples and the operational details of the reactor, including the use of a 'rabbit' capsule for irradiation.
🌟 Exploring Decay Mechanisms in Nuclear Science
This section focuses on the various mechanisms of radioactive decay, including positron emission, electron capture, and gamma decay. The dialogue explains these processes in detail, emphasizing the differences between them and the conditions under which they occur. The speakers also discuss the implications of these decay mechanisms for the study and application of nuclear science.
📈 Activity, Half-life, and Series Decay in Radioactive Elements
The paragraph introduces the concepts of activity and half-life in radioactive decay processes. It explains how these concepts are used to predict the behavior of radioactive substances over time. The discussion also touches on the idea of intentionally creating isotopes through neutron activation, a key aspect of Nuclear Activation Analysis.
🧠 Understanding the Mathematics of Radioactive Decay
This section presents a mathematical approach to understanding radioactive decay, focusing on the differential equations that describe the change in isotope concentrations over time. The speakers derive the exponential decay equation and discuss the relationship between decay constant and half-life. They also explore the concept of serial radioactive decay through a system of differential equations.
🔬 Modeling Nuclear Activation Analysis with Differential Equations
The paragraph discusses the process of modeling Nuclear Activation Analysis (NAA) using differential equations. It covers the creation and destruction rates of isotopes in a reactor due to neutron absorption and radioactive decay. The speakers provide a detailed explanation of how to set up these equations based on the decay and reaction mechanisms of the isotopes involved.
📉 Analyzing Complex Decay Diagrams and Equations
The speakers demonstrate how to analyze and construct a complex set of differential equations based on a decay diagram. They emphasize that regardless of the complexity, as long as the relationships between isotopes are clear, the equations can be formulated correctly. The paragraph concludes with an invitation for students to ask questions about the process.
Mindmap
Keywords
💡Nuclear Science Engineering
💡Computational Nature
💡Nuclear Reactor Lab
💡Nuclear Activation Analysis (NAA)
💡Radioactive Decay
💡Bateman Equations
💡Isotopes
💡Half-Life
💡Elemental Composition
💡Lab Activities
Highlights
The transcript discusses the misconception that nuclear science engineering is purely computational, emphasizing the importance of both theory and practical experiments.
MIT OpenCourseWare's commitment to offering high-quality educational resources for free is highlighted, with an encouragement for donations to support the initiative.
The introduction of a special assignment involving Nuclear Activation Analysis (NAA), a sensitive method for measuring impurities in materials, is presented.
Students are asked to bring in samples for NAA, prompting creative and unconventional sample selection, such as dog claws or food items, to understand elemental composition.
The use of nuclear reactors for educational purposes is explored, with students getting hands-on experience with irradiation processes.
The concept of isotopes and their decay, including the Bateman equations for radioactive decay, are discussed in the context of the assignment.
The practical aspects of handling nuclear samples, such as avoiding fissionable and overly salty materials, are emphasized for safety.
The educational use of nuclear reactors for irradiation experiments is highlighted, with students calculating the activation and decay of isotopes.
The naming convention in nuclear science, often related to animals and farm implements, is described, adding a lighter note to the technical discussion.
The application of NAA in environmental studies, such as analyzing atmospheric particulates, rainwater, and sediments for heavy metals, is covered.
The potential health risks of certain isotopes, like cobalt-60, and their importance in reactor core analysis are discussed.
The use of neutron activation analysis for determining the presence of specific elements in materials, even in trace amounts, is explained.
The transcript covers the process of irradiating samples in a nuclear reactor, emphasizing the safety protocols and procedures followed.
The educational experience of physically manipulating the power levels of a reactor and the significance of this hands-on approach are highlighted.
The importance of understanding the principles of radioactive decay, activity, and half-life for practical applications in nuclear engineering is underscored.
The concept of competing decay mechanisms, such as positron emission and electron capture, and their respective energy requirements are explained.
The educational value of conducting experiments with radioactive isotopes, like measuring the radioactivity of a large quantity of bananas, is discussed.
The use of the NIST X-ray tables for identifying and calculating the energy levels of X-rays emitted during radioactive decay processes is introduced.
The transcript concludes with a summary of the key takeaways from the lecture, reinforcing the connection between mass and energy in nuclear reactions.
Transcripts
Browse More Related Video

11. Radioactivity and Series Radioactive Decays
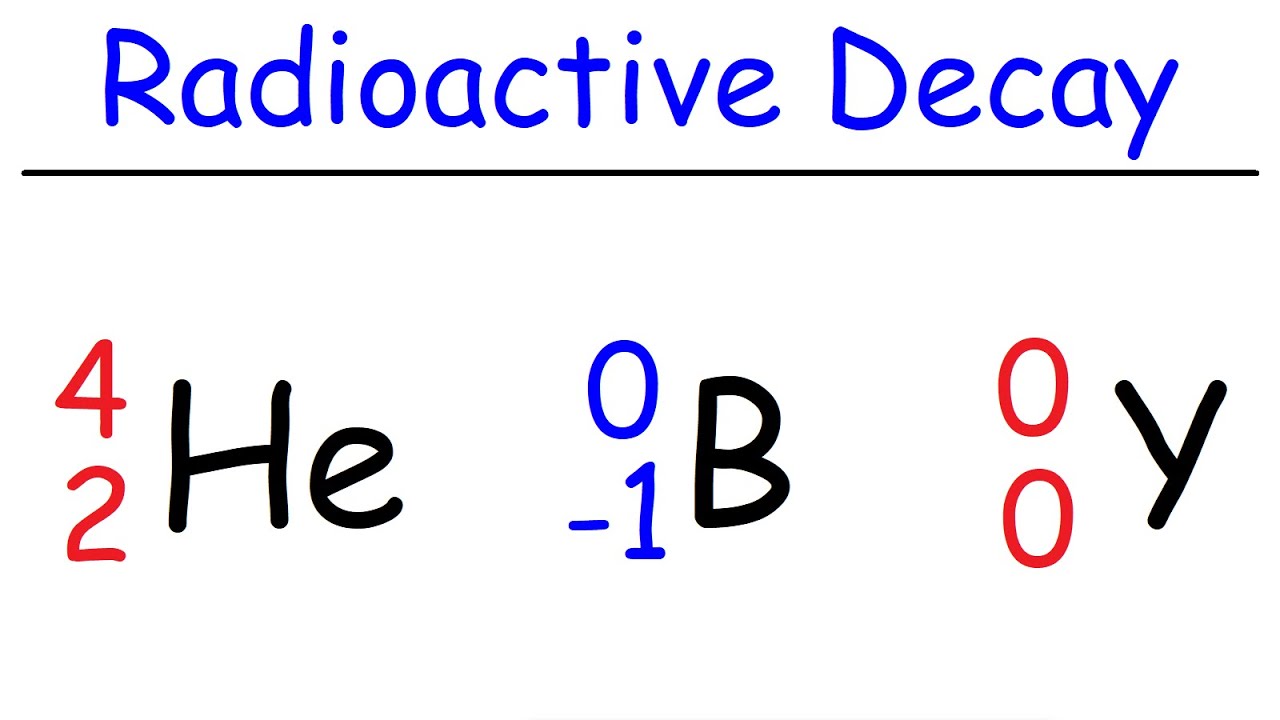
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry

12. Numerical Examples of Activity, Half Life, and Series Decay

8. Radioactive Decay — Modes, Energetics, and Trends

Alpha Particles, Beta Particles, Gamma Rays, Positrons, Electrons, Protons, and Neutrons

Why Does Everything Decay Into Lead
5.0 / 5 (0 votes)
Thanks for rating: