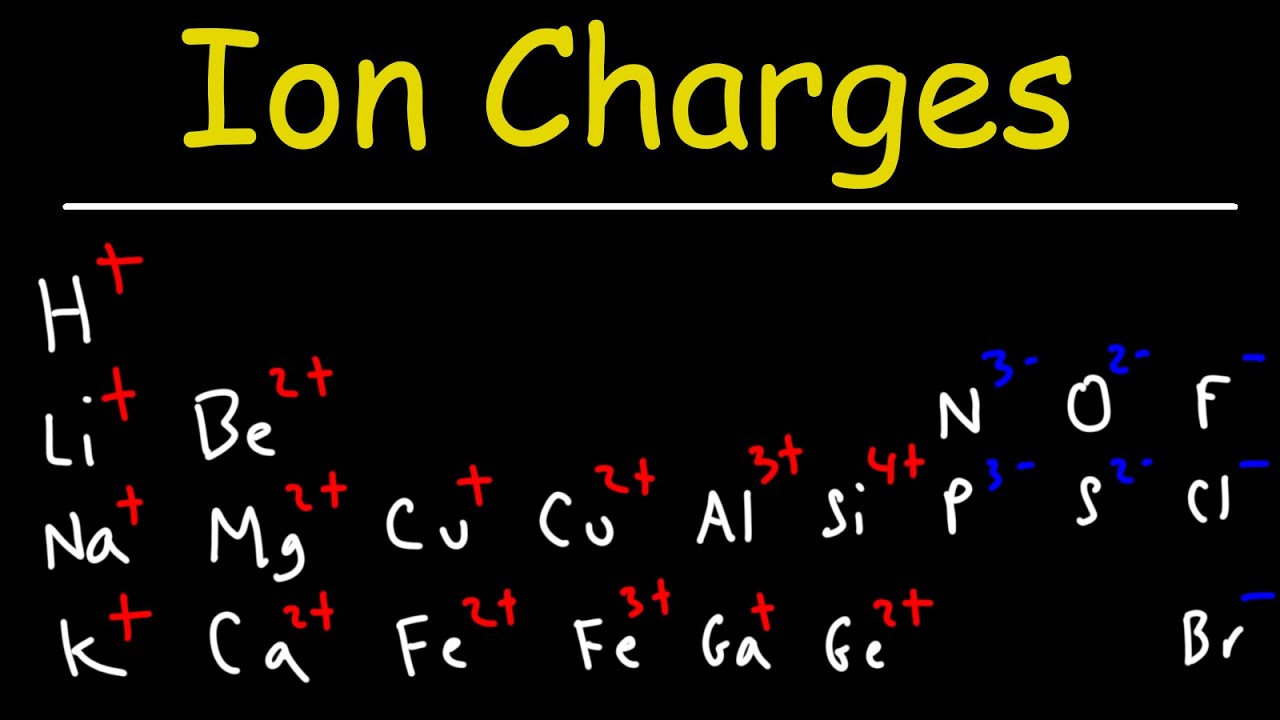Synthesis Reactions: Part 1 - Element + Element = Compound
TLDRThis video script introduces synthesis reactions, focusing on the combination of two elements to form a single compound. It explains the formation of ionic compounds by combining metals with non-metals, using the periodic table to determine the charge of each element and how to crisscross these charges to obtain the formula of the resulting compound. The script also addresses the unique behavior of pure elements like hydrogen, chlorine, and oxygen, and the formation of diatomic or cluster molecules. Furthermore, it discusses the variability in oxidation states of transition metals and non-metals, and how these can affect the outcome of synthesis reactions.
Takeaways
- 🧪 Synthesis reactions involve two substances combining to form a single product.
- 🔬 When a metal and a non-metal combine, they form an ionic compound.
- 📊 To determine the charge of elements in ionic compounds, one must consult the periodic table.
- 🔄 The charges of the combining elements are determined by their positions in the periodic table, such as aluminum (Al) having a +3 charge and fluorine (F) having a -1 charge.
- 🌐 By crisscrossing the charges, one can determine the formula of the ionic compound (e.g., AlF3 for aluminum fluoride).
- 🏗️ All ionic compounds are solids at room temperature, and the state symbol 's' can be used in chemical notation.
- 🎯 In synthesis reactions, the pure elemental form of some elements exists as diatomic molecules (e.g., Cl2, N2).
- 🤹♂️ Transition metals can have multiple possible charges, and the specific charge used in a reaction may depend on the reaction conditions or the teacher's preference.
- 🔥 Oxidation states of elements like carbon can vary, leading to different compounds such as carbon monoxide (CO) or carbon dioxide (CO2).
- 📚 Ionic compounds formed from synthesis reactions are represented by their elemental symbols and the corresponding charges.
- 📝 In cases where transition metals have multiple possible oxidation states, both possibilities may be acceptable unless specified otherwise by a teacher.
Q & A
What is a synthesis reaction?
-A synthesis reaction is a type of chemical reaction where two or more substances combine to form a single product.
What is the difference between an element and a compound?
-An element is a pure substance consisting of only one type of atom, while a compound is a pure substance composed of two or more different elements chemically bonded together.
How are ionic compounds formed?
-Ionic compounds are formed when a metal reacts with a non-metal, resulting in the transfer of electrons from the metal to the non-metal, creating positively and negatively charged ions that attract each other.
How can you determine the charge of an element in an ionic compound?
-You can determine the charge of an element in an ionic compound by consulting the periodic table and understanding the tendencies of elements based on their group and properties.
What is the state of ionic compounds at room temperature?
-All ionic compounds are solids at room temperature.
What does it mean when elements form diatomic molecules in their pure elemental form?
-When elements form diatomic molecules in their pure elemental form, it means that two atoms of the same element bond together to form a stable molecule. This is common for elements like hydrogen, nitrogen, and oxygen.
How can the charge of transition metals vary in synthesis reactions?
-The charge of transition metals can vary in synthesis reactions based on the amount of oxygen or non-metal present in the reaction. Different amounts can lead to different oxidation states of the metal.
What are two possible products of a reaction between copper and oxygen?
-Two possible products of a reaction between copper and oxygen are copper(I) oxide (Cu2O) and copper(II) oxide (CuO), depending on the amount of oxygen available.
What are the two forms of carbon oxides formed in combustion reactions?
-The two forms of carbon oxides formed in combustion reactions are carbon monoxide (CO) and carbon dioxide (CO2), which are products of incomplete and complete combustion, respectively.
How can the charge of cobalt affect the type of chloride it forms?
-The charge of cobalt can affect the type of chloride it forms by determining whether it forms cobalt(II) chloride (CoCl2) with a +2 charge or cobalt(III) chloride (CoCl3) with a +3 charge, depending on the amount of chlorine present in the reaction.
Outlines
🧪 Synthesis Reactions: Ionic Compounds Formation
This paragraph introduces synthesis reactions, focusing on the combination of two elements to form a single compound. It explains the formation of ionic compounds when a metal reacts with a non-metal, emphasizing the importance of understanding the charges each atom acquires based on their position in the periodic table. The process of crisscrossing charges to determine the formula of ionic compounds is detailed, using examples like aluminum fluoride (AlF3), calcium nitride (Ca3N2), lithium oxide (Li2O), and potassium fluoride (KF). The paragraph also touches on the concept of pure elements and diatomic molecules, and how transition metals can form compounds with varying charges depending on the reaction's conditions.
🌐 Synthesis Reactions Continued: Transition Metals and Oxides
The second paragraph continues the discussion on synthesis reactions, particularly focusing on the formation of oxides and the role of transition metals. It explains how the availability of oxygen can affect the charge and, consequently, the type of compound formed. The paragraph uses examples of iron (Fe) and cobalt (Co) to illustrate how different charges can lead to different compounds, such as FeO and Fe2O3 (rust), and CoCl2 and CoCl3. Additionally, it mentions the formation of carbon monoxide and carbon dioxide through combustion reactions, which can also be considered synthesis reactions when forming pure carbon compounds. The summary emphasizes the variability in the outcome of synthesis reactions involving transition metals and the importance of following specific guidance provided by teachers in academic settings.
Mindmap
Keywords
💡Synthesis reactions
💡Ionic compounds
💡Periodic table
💡Charges
💡Crisscross method
💡Transition metals
💡Diatomic molecules
💡Clusters
💡Combustion reactions
💡Stoichiometry
💡Chemical symbols
Highlights
Introduction to synthesis reactions as a fundamental concept in chemistry.
Explanation of how two elements combine to form a compound, specifically focusing on metal and non-metal combinations resulting in ionic compounds.
Use of the periodic table to determine the charge preference of elements in ionic compounds, such as aluminum and fluorine.
The concept of crisscrossing charges to derive the formula of an ionic compound, exemplified by aluminum fluoride (AlF3).
Discussion on the general state of ionic compounds at room temperature, noting that they are typically solids.
Demonstration of how to determine the formula for calcium nitride (Ca3N2) through charge crisscrossing.
Explanation of lithium oxide (Li2O) formation and the process of charge crisscrossing for lithium and oxygen.
Introduction to the concept of pure elements and their diatomic molecular form in nature, such as hydrogen and oxygen.
Discussion on transition metals and their potential for multiple charges, and how the surrounding elements and conditions can influence the final compound formed.
Illustration of possible compounds formed from copper and oxygen, including copper(I) oxide (Cu2O) and copper(II) oxide (CuO).
Explanation of how the charge of iron can vary in compounds, leading to different oxidation states such as iron(II) oxide (FeO) and iron(III) oxide (Fe2O3).
Introduction to the formation of carbon monoxide and carbon dioxide from carbon and oxygen, relating to combustion reactions.
Clarification on the notation for pure metal element symbols in the periodic table.
Guidance on handling transition metals with multiple possible charges, suggesting the need for teacher's direction or considering both possibilities.
Review and summary of the key points discussed about synthesis reactions, including the combination of elements, formation of ionic compounds, and the role of the periodic table.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: