Ch#24 |Lec#3 || INFRARED SPECTROSCOPY (IR SPECTROSCOPY) CLASS12
TLDRThe script discusses the principles and applications of infrared spectroscopy in studying molecular vibrations and identifying functional groups within organic compounds. It explains how different functional groups absorb infrared radiation, resulting in specific peaks in the spectrum that can be used for identification. The concepts of transmission percentage, fingerprint region, and the importance of recognizing strong peaks for structural identification are highlighted, emphasizing the technique's utility in understanding molecular structure and composition.
Takeaways
- 🌟 Infrared spectroscopy is a technique used to study the electromagnetic radiation emitted by molecules, providing insights into their structure and composition.
- 🔍 The process involves analyzing the absorption spectrum of a compound, which reveals the presence of specific functional groups and bonds within the molecule.
- 📈 The spectrum is represented as a graph with wavenumber (cm⁻¹) on the x-axis and percent transmittance on the y-axis, allowing for the identification of characteristic peaks.
- 🏠 Molecules with different functional groups exhibit distinct absorption patterns, known as 'fingerprint regions,' which can be compared to a reference table for identification.
- 📊 The intensity of peaks in the spectrum can vary, with stronger peaks indicating a higher concentration of a particular bond or functional group within the molecule.
- 🔬 Vibrational spectroscopy is a type of infrared spectroscopy that focuses on the vibrational energy levels of molecules, which change when they absorb infrared radiation.
- 🎵 Different types of molecular vibrations include stretching, bending, and rocking, each of which corresponds to specific regions in the infrared spectrum.
- 🌈 The concept of 'fingerprint region' is crucial for identifying compounds, as each organic compound has a unique fingerprint spectrum that differs from others.
- 🔍 The combination of functional group regions and fingerprint regions in the spectrum allows for the detailed identification of the structure of organic compounds.
- 📚 Understanding the principles of infrared spectroscopy and the interpretation of spectra is essential for studying the properties and interactions of molecules in various fields of science.
Q & A
What is the basic technique discussed in the script for studying a substance?
-The basic technique discussed in the script is Infrared Spectroscopy, which is used to study a substance by analyzing the electromagnetic radiation absorbed or emitted by the molecular vibrations within the substance.
What does the term 'Y Spectrum Copy' refer to in the context of the script?
-The term 'Y Spectrum Copy' refers to Infrared Spectroscopy, a method used to study the molecular structure of a substance by examining the specific wavelengths of infrared light absorbed or emitted by the sample.
What is the role of a spectrophotometer in this technique?
-A spectrophotometer is an instrument used in Infrared Spectroscopy to measure the intensity of light at different wavelengths, allowing the identification of specific functional groups and bonds within a compound based on their absorption or emission characteristics.
How does the script explain the identification of functional groups in a compound?
-The script explains that by analyzing the spectrum, one can identify the presence of specific functional groups within a compound. This is done by recognizing the characteristic absorption or emission peaks that correspond to particular bond vibrations, such as carbon-carbon, carbon-hydrogen, or carbon-nitrogen bonds.
What is the significance of the literature data provided to the students?
-The literature data, typically in the form of tables, provides a reference for students to compare the spectral data obtained from experiments. It helps in identifying the functional groups and bonds present in a compound by comparing the observed peaks with the known values in the literature.
What is the wavelength range of the infrared region studied in Infrared Spectroscopy?
-The wavelength range of the infrared region studied in Infrared Spectroscopy is typically from 2.5 to 16 micrometers, which corresponds to the wavenumber range of 4000 to 625 cm^-1.
How does the script differentiate between 'Fingerprint Region' and 'Functional Group Region' in the spectrum?
-The script differentiates the two regions by their wavenumber ranges. The 'Fingerprint Region' typically ranges from 600 to 1500 cm^-1, while the 'Functional Group Region' extends beyond 1500 cm^-1. The 'Fingerprint Region' is characterized by numerous peaks that are unique to each compound, whereas the 'Functional Group Region' shows fewer, more intense peaks corresponding to specific functional groups.
What is the importance of understanding vibrational motions in molecules for Infrared Spectroscopy?
-Understanding vibrational motions in molecules is crucial for Infrared Spectroscopy because these vibrations lead to changes in the dipole moment of the molecule, which in turn affects the absorption or emission of infrared radiation. By studying these changes, one can gain insights into the molecular structure and the types of bonds and functional groups present.
How does the script describe the process of identifying a compound's structure using Infrared Spectroscopy?
-The script describes the process as one that involves comparing the observed spectral peaks with the literature data to identify the functional groups and bonds present in the compound. This involves recognizing the characteristic peaks associated with different types of bonds and using this information to deduce the compound's structure.
What are the different types of vibrations that can occur in a molecule as explained in the script?
-The script explains several types of molecular vibrations, including stretching vibrations, where the bond length changes but the bond angle remains constant; bending vibrations, where the bond angle changes; and twisting and rocking vibrations, which involve more complex motion patterns within the molecule.
Outlines
🔬 Introduction to Infrared Spectroscopy
This paragraph introduces the concept of infrared spectroscopy, emphasizing its use in studying molecular vibrations within compounds. It explains the basic technique of using a spectrophotometer to analyze the absorption of infrared radiation by a sample, which provides information about the functional groups and chemical bonds present. The paragraph also touches on the importance of understanding the literature and data tables provided in the study materials, which help in identifying the functional groups and bonds within organic compounds through their spectral data.
📊 Principles and Applications of Infrared Spectroscopy
The second paragraph delves into the fundamental principles of infrared spectroscopy, highlighting how molecular vibrations within a molecule change when exposed to infrared radiation. It discusses the concept of active substances and how their vibrations are affected by the radiation, leading to changes in the molecule's dipole moment and polarizability. The paragraph also explains the process of identifying functional groups and chemical bonds in a compound using an absorption spectrum, and how to compare this with literature data to confirm the structure of the compound.
🌐 Types of Molecular Vibrations in Infrared Spectroscopy
This paragraph focuses on the different types of molecular vibrations that can be observed in infrared spectroscopy, such as stretching, bending, and rocking vibrations. It describes how these vibrations relate to changes in bond length and bond angles within the molecule. The paragraph also explains the concept of symmetric and asymmetric vibrations, and how they manifest in the infrared spectrum. The discussion extends to the identification of functional groups and the interpretation of the fingerprint region, which is unique to each compound.
📈 Interpreting Infrared Spectra for Structural Identification
The final paragraph discusses the process of interpreting infrared spectra to identify the structure of organic compounds. It explains how the presence of strong peaks in the spectrum can indicate specific functional groups, such as carbon-hydrogen bonds. The paragraph also emphasizes the importance of considering the percentage transmittance and how it relates to the intensity of absorption. It concludes by reinforcing the idea that infrared spectroscopy is a powerful tool for determining the structure of compounds by analyzing the unique patterns of absorption and comparing them to known data.
Mindmap
Keywords
💡Infrared Spectroscopy
💡Functional Groups
💡Spectral Analysis
💡Wavelength
💡Peaks
💡Molecular Structure
💡Absorption
💡Vibrational Spectroscopy
💡Spectral Table
💡Wavenumber
💡Transmittance
Highlights
The lecture introduces the concept of infrared spectroscopy and its application in studying molecular vibrations.
Infrared spectroscopy is a technique used to analyze the specific frequencies absorbed by a molecule, which helps in identifying functional groups and bonds within the compound.
The use of a spectrometer is explained as a fundamental instrument for detecting electromagnetic radiation emitted or absorbed by molecules.
The lecture emphasizes the importance of understanding basic instrument techniques, such as the use of a spectrometer, for studying spectroscopy.
Students are introduced to the concept of functional groups within organic compounds and how they can be identified using infrared spectroscopy.
The role of literature in providing data and tables for understanding the relationship between peak positions and bond types in a spectrum is highlighted.
The lecture explains how to interpret an infrared spectrum by identifying the functional groups and bonds present in a compound based on peak positions and values.
The concept of wavenumber range and its significance in identifying the type of bonds and functional groups in a compound is discussed.
Vibrational spectroscopy is introduced as a type of infrared spectroscopy that studies the vibrational motion within molecules when affected by radiation.
The lecture explains the basic principle of infrared spectroscopy, which involves the change in vibrational energy of molecules when they absorb electromagnetic radiation.
The difference between various types of molecular vibrations, such as stretching, bending, and rocking, is clarified in the context of infrared spectroscopy.
The concept of fingerprint region in spectroscopy and its importance in identifying unique compounds is discussed.
The lecture provides a method for identifying the structure of organic compounds by comparing the spectrum with literature data and identifying the functional groups and bonds.
The significance of understanding the wavenumber range of different functional groups for their identification in spectroscopy is emphasized.
The lecture concludes with a brief overview of how to use the lecture content for effective learning and application in spectroscopy.
Transcripts
Browse More Related Video

Functional Groups from Infrared Spectra

Introduction to IR Spectroscopy: How to Read an Infrared Spectroscopy Graph

14.1 Introduction to IR Spectroscopy | Organic Chemistry

IR Spectroscopy and Mass Spectrometry: Crash Course Organic Chemistry #5

IR Spectroscopy
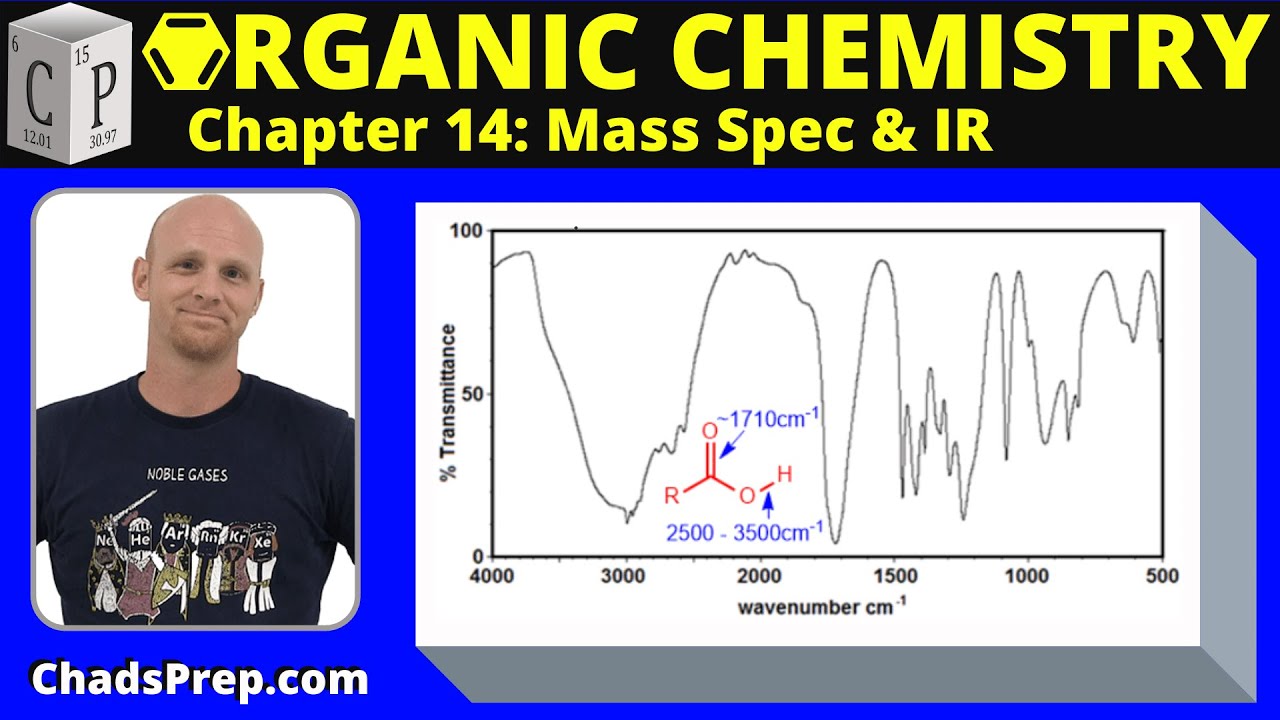
14.2a IR Spectra of Carbonyl Compounds | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: