Functional Groups from Infrared Spectra
TLDRInfrared spectroscopy is a powerful analytical tool used to identify functional groups within molecules without revealing their connectivity. The technique involves examining specific regions of the infrared spectrum: above 3000 cm⁻¹ for O-H, N-H, and sp²/sp hybridized carbon-hydrogen stretches; 2500-2000 cm⁻¹ for carbon-carbon and carbon-nitrogen triple bonds; and 1500 cm⁻¹ onwards for fingerprint region, CC, CN, and CO stretches. The script illustrates how to analyze an infrared spectrum by identifying key peaks and their corresponding functional groups, such as hydroxyl, nitrile, carboxilic acid, and aromatic structures. It also highlights the importance of recognizing sp³ hybridized carbons and the presence of primary amines, which are indicative of specific molecular structures. The examples provided in the script demonstrate the identification of hydroxy propionitrile, a carboxylic acid, and benzylamine, showcasing the practical application of infrared spectroscopy in structural elucidation.
Takeaways
- 🔬 **Infrared Spectroscopy Overview**: Infrared spectroscopy is used to identify functional groups within molecules without revealing their connectivity.
- 📈 **Spectrum Analysis Approach**: When analyzing an infrared spectrum, start with a broad view and then focus on key spectral elements.
- 🌈 **Spectral Region Division**: The infrared spectrum is divided into specific regions based on the types of bonds and stretches present (O-H, N-H, C-H, etc.).
- 🔍 **Identifying Hydroxyl Groups**: A large peak outside 3000 cm⁻¹ is indicative of an O-H stretch, suggesting a hydroxyl group in the molecule.
- 📉 **SP3 Hybridized Carbons**: The absence of CH stretches beyond 3000 cm⁻¹ implies the presence of only sp3 hybridized carbons in the structure.
- 🔍 **Nitrile Group Identification**: A peak in the 2000-2500 cm⁻¹ range, particularly on the higher end, suggests a nitrile (C≡N) group.
- 🔑 **Fingerprint Region**: The region below 1500 cm⁻¹ is often used for identifying specific stretches of CC, CN, and CO bonds, though it can be less diagnostic.
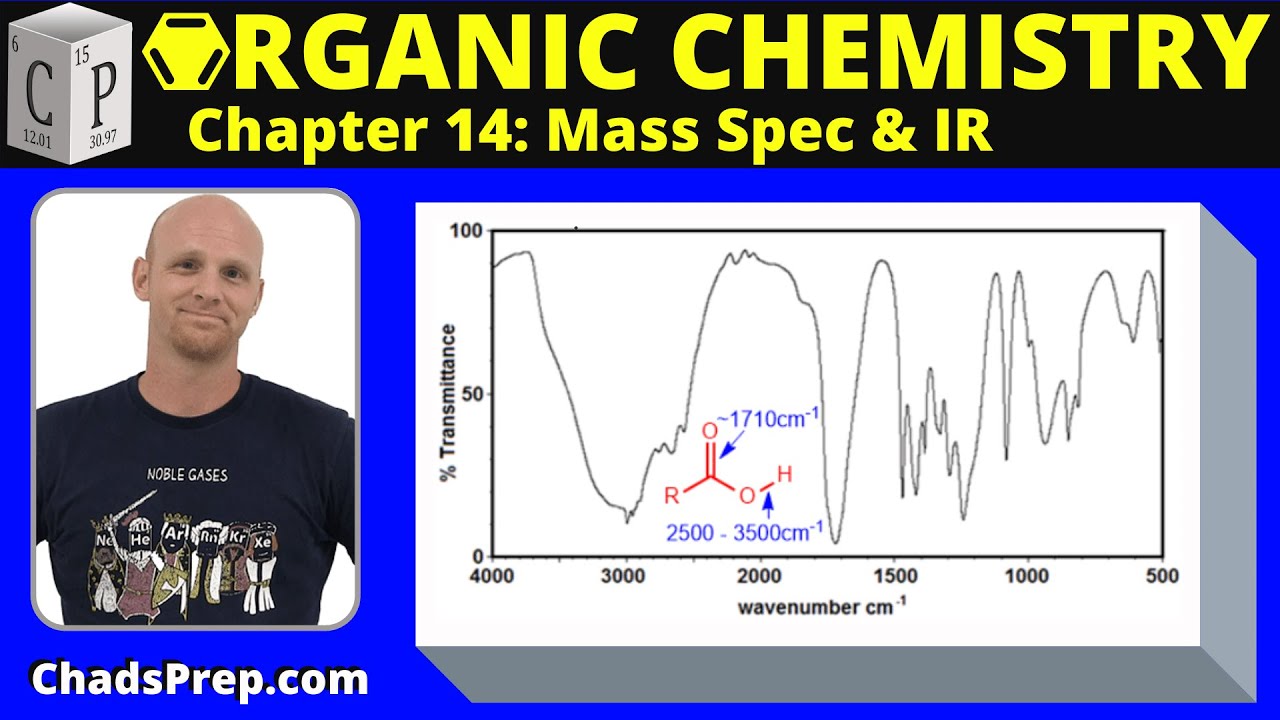
- 🧪 **Alcoholic and Carboxylic Acids**: Broad peaks around 3000 cm⁻¹ and a strong peak around 1700 cm⁻¹ can indicate the presence of hydroxyl and carboxyl groups, respectively.
- 🔍 **Primary Amine Identification**: Two peaks in the NH stretching region suggest the presence of a primary amine with two NH bonds.
- 🌟 **Aromatic Structure Indicators**: Strong peaks in the fingerprint region, particularly around 1450-1600 cm⁻¹, are indicative of an aromatic structure with sp2 hybridized carbons.
- 📝 **Combining Observations**: By combining observations from different regions of the spectrum, one can deduce the structure of the compound, such as identifying a benzylamine with a primary amine and a mono-substituted benzene ring.
Q & A
What is the primary purpose of infrared spectroscopy?
-Infrared spectroscopy is used to identify the functional groups within a molecule without providing information on how these groups are connected to each other.
How should one approach infrared spectroscopy problems?
-One should start with the big picture, then focus on the important elements of a spectrum by dividing the infrared region into specific regions.
What types of bonds are typically found above 3000 cm⁻¹ in an infrared spectrum?
-Above 3000 cm⁻¹, one typically finds O-H, N-H, and the SP2 and SP hybridized carbon-hydrogen stretches.
What does the absence of CH stretches beyond 3000 cm⁻¹ indicate about the structure?
-The absence of CH stretches beyond 3000 cm⁻¹ indicates that the structure contains only sp³ hybridized carbons.
What is the significance of a peak around 1700 cm⁻¹ in an infrared spectrum?
-A peak around 1700 cm⁻¹ is characteristic of a carbonyl (C=O) group, suggesting the presence of a carboxyl (COOH) functionality in the molecule.
What does the presence of a strong peak around 3000 cm⁻¹ indicate?
-A strong peak around 3000 cm⁻¹ is indicative of an O-H group, suggesting the presence of a hydroxyl group in the structure.
What is the typical appearance of a primary amine in an infrared spectrum?
-A primary amine, which contains two N-H bonds, will show two peaks in the N-H stretching region of the spectrum.
What does the presence of peaks around 1450 to 1600 cm⁻¹ suggest about the structure?
-The presence of peaks in the region of 1450 to 1600 cm⁻¹ is indicative of an aromatic structure, suggesting SP2 hybridized carbons bonded to hydrogen.
What is the fingerprint region in an infrared spectrum and why is it important?
-The fingerprint region is below 1500 cm⁻¹ and consists mainly of medium to weak peaks. It is important because it can provide unique structural information that helps in identifying the compound.
What are the key features to look for when identifying a nitrile group in an infrared spectrum?
-The key feature is a peak that is more squarely in the range of a nitrile, typically on the higher end of the 2000 to 2500 cm⁻¹ range, indicating the presence of a CN triple bond.
How can one differentiate between an alkene and a nitrile using infrared spectroscopy?
-Alkenes typically show double bond stretches around 3300 cm⁻¹, while nitriles show a characteristic peak in the higher end of the 2000 to 2500 cm⁻¹ range due to the CN triple bond.
What is the significance of the peak around 2500 cm⁻¹ in an infrared spectrum?
-A peak around 2500 cm⁻¹ is typically indicative of triple bond stretches, such as carbon-carbon or carbon-nitrogen triple bonds in the molecule.
Outlines
🌟 Infrared Spectroscopy: Identifying Functional Groups
Infrared spectroscopy is a technique that allows the identification of functional groups within molecules without revealing their connectivity. The process involves examining the spectrum's significant elements by dividing the infrared region into specific zones. Key regions include above 3000 cm⁻¹ for O-H, N-H, and sp²/sp hybridized carbon-hydrogen stretches; 2500-2000 cm⁻¹ for carbon-carbon and carbon-nitrogen triple bonds; and 1500 cm⁻¹ onwards for fingerprint region or specific stretches of CC, CN, and CO that are less diagnostic. The script discusses the analysis of a spectrum with a prominent peak outside 3000 cm⁻¹, indicating an O-H group, and the absence of CH stretches beyond 3000 cm⁻¹, suggesting sp³ hybridized carbons. It also highlights a peak in the 2000-2500 cm⁻¹ range, likely corresponding to a nitrile group. The compound in question is hydroxy propionitrile, containing both hydroxyl and nitrile groups. The method is applied to identify functional groups in different examples, including an alkane or nitrile with a peak in the 2000-2500 cm⁻¹ range, and an aldehyde or carboxylic acid with a peak around 1700 cm⁻¹. The script emphasizes the importance of recognizing peaks associated with specific functional groups and using the spectrum's divisions to draw conclusions about molecular structure.
🔍 Analyzing Infrared Spectrum for Structural Features
This paragraph delves into the analysis of another infrared spectrum to identify functional groups. The spectrum is divided into specific regions: the 3000 cm⁻¹ line for O-H and N-H stretches, the 2000-2500 cm⁻¹ range for triple bonds, the 2500 cm⁻¹ line for double bonds, and the region below 1500 cm⁻¹ for fingerprint and heavy atom single bond stretches. A broad peak near 3000 cm⁻¹ is indicative of an O-H group, while peaks just below 3000 cm⁻¹ suggest sp³ hybridized carbons bonded to hydrogens. A strong and clear peak around 1700 cm⁻¹ is associated with a carbonyl group, particularly when a hydroxyl group is also present, indicating a carboxylic acid functionality. The fingerprint region's medium to weak peaks provide less definitive information. The compound's true structure is identified as an aliphatic carboxylic acid, containing only sp³ hybridized carbons, carbon-carbon single bonds, and a carboxylic acid functional group. In the final example, the spectrum shows activity in the fingerprint region and typical peaks for CH stretches. Notably, two peaks indicative of an O-H or N-H group suggest a primary amine's presence, confirmed by the two-pronged appearance in the N-H stretching region. The presence of an aromatic structure is inferred from strong peaks in the fingerprint region around 1450-1600 cm⁻¹, consistent with SP² hybridized carbons bonded to hydrogen. The structure is confirmed as benzylamine, which includes a primary amine, an sp³ hybridized CH₂ group, and a mono-substituted benzene ring.
Mindmap
Keywords
💡Infrared Spectroscopy
💡Functional Groups
💡Hydroxy Group
💡Nitrile Group
💡Sp3 Hybridized Carbons
💡Triple Bonds
💡Double Bonds
💡Fingerprint Region
💡Primary Amine
💡Aromatic Structure
💡Benzylamine
Highlights
Infrared spectroscopy is a tool to identify functional groups within a molecule without knowing their connectivity.
Approach infrared spectroscopy problems by starting with the big picture and then focusing on important elements of the spectrum.
Divide the infrared region of the spectrum into specific regions: above 3,000 for O, NH and SP2/SP hybridized carbon-hydrogen stretches; 2,500-2,000 for carbon-carbon, carbon-nitrogen triple bond stretches; 2,500 for double bond stretches; below 1,500 for fingerprint region or specific CC, CN, CO stretches.
A large peak outside 3,000 indicates the presence of an O group.
No CH stretches beyond 3,000 implies only SP3 hybridized carbons are present.
A peak in the 2,000-2,500 range is characteristic of an alkyne or nitrile, with a nitrile being more likely on the higher end.
The compound in the first example is hydroxy propionitrile, containing both hydroxyl and nitrile groups.
A very broad peak around 3,000 wave numbers indicates an O group.
Peaks just south of 3,000 are indicative of SP3 hybridized carbons bonded to hydrogens.
A strong peak around 1,700, along with a hydroxyl group, suggests the presence of a carboxylic acid functionality.
The true structure of the second compound is an aliphatic carboxylic acid, containing only C-H, C-C single bonds and a carboxylic acid group.
In the third example, peaks around 3,000 are typical for CH stretches, while peaks north of 3,000 indicate SP2 hybridized carbon-hydrogen stretches.
Two peaks indicative of an O or NH in the NH stretching region suggest the presence of a primary amine.
A primary amine with two NH bonds will show two peaks in the NH stretching region.
Strong peaks in the fingerprint region around 1,450-1,600 are indicative of an aromatic structure.
The presence of an aromatic structure goes well with SP2 hybridized carbons bonded to hydrogen.
The true structure of the third compound is benzylamine, containing a primary amine, a CH2 group (origin of SP3 hybridized carbon-hydrogen stretches), and a monosubstituted benzene ring.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: