Chemical Equilibria (Contd.)
TLDRThe lecture delves into the concept of chemical equilibrium, emphasizing its relevance in analytical chemistry. It discusses the auto-protolysis of water, the behavior of acids and bases, and the distinction between strong and weak acids. The role of the solvent in proton donation and acceptance is highlighted, with examples like hydrochloric acid and perchloric acid. The lecture further explores the equilibrium in redox reactions, using the reaction between arsenate and iodide ions as an illustrative example. It touches on the Le Chatelier principle and the impact of temperature and pressure on equilibrium, concluding with a discussion on equilibrium constants and their calculation.
Takeaways
- 📚 The class focuses on chemical equilibrium, a concept crucial for future analytical chemistry studies.
- 💧 The discussion begins with the equilibrium of water and its autoprotolysis, forming H3O+ and OH- ions.
- 🔄 The amphiprotic nature of water allows it to act as both an acid and a base in different reactions.
- 🧪 The strength of acids and bases is influenced by their ability to donate or accept protons, with strong acids completely dissociating in water.
- 🥼 The role of the solvent in chemical equilibrium is highlighted, with water being an effective solvent and proton acceptor.
- 🌡️ Changes in temperature and pressure can shift the position of the equilibrium according to Le Chatelier's principle.
- 📊 The equilibrium constant (K) is introduced as a measure of the extent of a reaction at equilibrium.
- 🌟 The concept of simultaneous and competitive equilibria is mentioned, with different reactions potentially occurring at the same time.
- 🥊 The strength of acids is further explained through the comparison of hydrochloric acid (HCl) and perchloric acid (HClO4) in aqueous and anhydrous conditions.
- 📈 The importance of understanding the equilibrium constant (K) in predicting the direction and extent of a reaction is emphasized.
- 🔬 The practical application of chemical equilibrium is illustrated through the example of an oxidation-reduction reaction involving arsenate and iodide ions.
Q & A
What is the main topic of discussion in the provided transcript?
-The main topic of discussion in the transcript is chemical equilibrium, specifically focusing on its properties, concepts, and its application in analytical chemistry.
Why is chemical equilibrium important in the context of analysis?
-Chemical equilibrium is important in analysis because it allows chemists to predict and understand the behavior of reactions under various conditions, which is crucial for accurate experimental results and data interpretation.
What is the significance of the water molecule (H2O) in the context of chemical equilibrium?
-Water molecules play a significant role in chemical equilibrium because they can act as both a proton donor (acid) and a proton acceptor (base), leading to the formation of species like H3O+ and OH-, which are essential in acid-base equilibria.
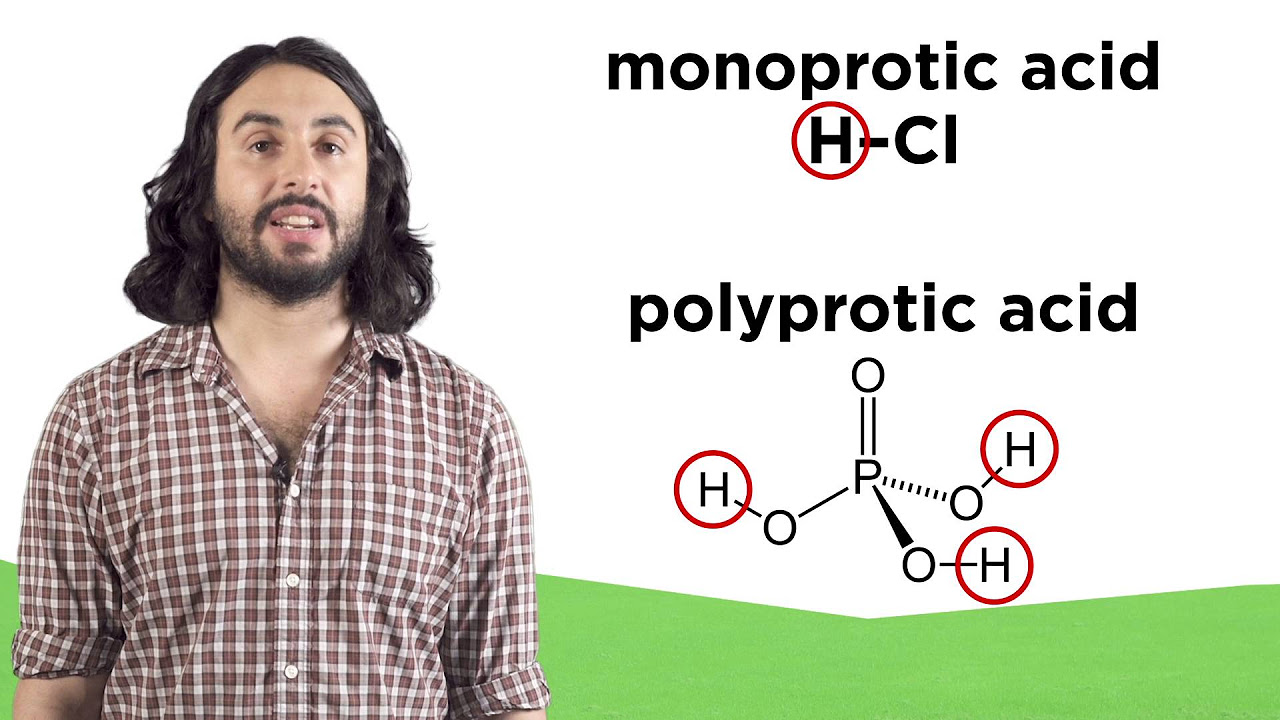
How does the strength of an acid affect its dissociation in water?
-The strength of an acid determines the extent to which it dissociates in water. Strong acids, like hydrochloric acid (HCl), dissociate completely, while weak acids do not, resulting in a partial dissociation and establishing an equilibrium between the undissociated acid and its ions.
What is the role of the solvent in acid-base equilibrium?
-The solvent plays a crucial role in acid-base equilibrium by influencing the dissociation of acids and bases. The solvent's ability to accept or donate protons can affect the strength of the acid or base, with water being a commonly used solvent that facilitates proton transfer.
How does the concept of amphiproticity relate to the autoprotolysis of water?
-Amphiproticity refers to the ability of a species to act as both an acid and a base. In the context of water, autoprotolysis is an example of amphiproticity where water molecules can donate protons to other water molecules, forming H3O+ and OH- ions.
What is the difference between strong and weak acids in terms of their behavior in anhydrous conditions?
-In anhydrous conditions, where water is absent, both strong and weak acids do not dissociate completely. The distinction between strong and weak acids based on their degree of dissociation in aqueous solutions becomes less relevant, as neither can fully dissociate without the presence of a proton-accepting solvent like water.
How can the equilibrium constant (K) be used to predict the extent of a reaction at equilibrium?
-The equilibrium constant (K) provides a measure of the extent of a reaction at equilibrium. A large K value indicates that the reaction favors the formation of products, while a small K value suggests that the reaction favors the reactants. By comparing the K value to 1, we can predict whether the reaction will predominantly proceed in the forward or reverse direction at equilibrium.
What is the Le Chatelier's principle, and how does it relate to chemical equilibrium?
-Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, such as temperature, pressure, or concentration of reactants or products, the position of equilibrium moves to counteract the change. This principle helps predict how the equilibrium will shift in response to external changes, allowing chemists to control the outcomes of reactions.
What is the significance of the mass action effect in controlling chemical equilibrium?
-The mass action effect is the principle that the rate of a chemical reaction depends on the concentration of the reactants. It plays a crucial role in controlling chemical equilibrium by determining the concentrations of reactants and products at equilibrium. This effect is particularly important when calculating equilibrium constants and predicting shifts in equilibrium due to changes in concentration.
How can the color change of certain species, such as tri-iodide ions, be used to visually monitor the progress of a reaction?
-The color change of species like tri-iodide ions (I3-) can be used to visually monitor the progress of a reaction because these species have a distinctive color that changes as the reaction proceeds. For example, the formation of I3- from iodide ions (I-) in the presence of arsenic acid can be tracked by observing the development of the characteristic red-brown color of I3-. This visual monitoring can be supplemented with spectrophotometric measurements for precise concentration determinations.
Outlines
📘 Introduction to Analytical Chemistry and Chemical Equilibrium
This paragraph introduces the class on analytical chemistry with a focus on chemical equilibrium. It explains the importance of understanding the properties and concepts of chemical equilibrium for future analysis. The discussion begins with a simple reaction involving water and its amphiprotic nature, forming H3O+ and HO- ions. The concept of acids and bases, and how they donate and accept protons, is introduced. The behavior of water and methanol as both acids and bases is also explained, highlighting the self-ionization of water.
🧪 Strong and Weak Acids: Dissociation and Solvent Effects
This paragraph delves into the distinction between strong and weak acids, using hydrochloric acid as an example of a strong acid that dissociates completely in water, while weak acids do not. The role of the solvent in accepting or donating protons and its impact on the strength of acids and bases is discussed. The equilibrium between the forward and reverse reactions and how it can be shifted by the solvent's properties is also explored, with a comparison between aqueous and anhydrous conditions for acids like hydrochloric acid and perchloric acid.
🌟 Redox Reactions and Chemical Equilibrium
This paragraph introduces the concept of redox reactions and their relation to chemical equilibrium. An example involving the reaction between arsenate ions and iodide ions is used to illustrate how the oxidation states of species change during the reaction. The equilibrium between reactants and products is visually observable through the formation of the colored tri-iodide ion. The rate and extent of the reaction are discussed, with emphasis on how the equilibrium constant (K value) can be used to understand the position of equilibrium and how changes in conditions like temperature and pressure can affect it.
🔄 Le Chatelier's Principle and Equilibrium Constants
This paragraph discusses Le Chatelier's Principle, which states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change. The effect of temperature and pressure on the position of equilibrium is explained, using the example of ammonia production through the Haber process. The concept of equilibrium constants (KA value) is introduced, explaining how it relates to the concentrations of reactants and products at equilibrium. The paragraph concludes by noting that solids and liquids are considered to have a concentration of unity in these calculations.
📚 Summary and Future Applications of Acid-Base Equilibrium
The final paragraph summarizes the key points discussed in the class, including the concepts of strong and weak acids, the role of solvents, redox reactions, and the importance of equilibrium constants. It also hints at future classes where the establishment of equilibrium values for acid-base reactions will be explored. The paragraph emphasizes the practical applications of understanding these chemical principles in analytical chemistry.
Mindmap
Keywords
💡Chemical Equilibrium
💡Acid
💡Base
💡Protons
💡Conjugate Acid-Base Pairs
💡Dissociation
💡Solvent
💡Redox Reaction
💡Equilibrium Constant (K)
💡Le Chatelier's Principle
💡Iodometric Titration
Highlights
Introduction to the concept of chemical equilibrium and its importance in analytical chemistry.
Discussion on the auto-protolysis of water and the formation of H3O+ and OH- ions.
Explanation of how water can act as both an acid and a base in certain reactions.
Comparison between strong acids like HCl and weak acids like acetic acid in terms of their dissociation in water.
The role of the solvent in determining the strength of acids and bases, and how it affects dissociation.
The concept of non-aqueous solutions and how they can alter the behavior of acids and bases.
Example of a redox reaction involving arsenate and iodide ions to illustrate chemical equilibrium.
Visual indication of equilibrium through the formation of tri-iodide ions and their color change.
Explanation of how the position of equilibrium can be affected by changes in temperature and pressure.
Introduction to the Le Chatelier's principle and its implications on chemical equilibrium.
Discussion on the calculation of equilibrium constants (K) and their significance in understanding reaction dynamics.
Mention of the Haber's process for ammonia production as an industrial application of equilibrium principles.
Explanation of how simultaneous equilibria can occur with a common species involved in multiple reactions.
The importance of using activities instead of concentrations for more accurate equilibrium calculations.
The impact of external conditions on the position of equilibrium and the mass action effect.
The significance of understanding chemical equilibrium in analytical procedures like iodometric titrations.
The dynamic nature of chemical equilibrium and how it can be shifted by altering reaction conditions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: