Nuclide Symbols: Atomic Number, Mass Number, Ions, and Isotopes
TLDRProfessor Dave enlightens viewers on the fundamentals of nuclide symbols, explaining the composition of atoms, the significance of protons in determining elements, and the concept of atomic and mass numbers. He delves into isotopes, detailing how they vary in neutron count, and ions, which are atoms with differing electron counts. The video also addresses the concept of average atomic masses on the periodic table, attributing the decimal values to the weighted average of isotopes based on their natural abundance.
Takeaways
- 🌟 Atoms consist of a nucleus with protons and neutrons, and electrons orbiting at a distance.
- 🚩 The proton number defines the element, with elements arranged by atomic number in the periodic table.
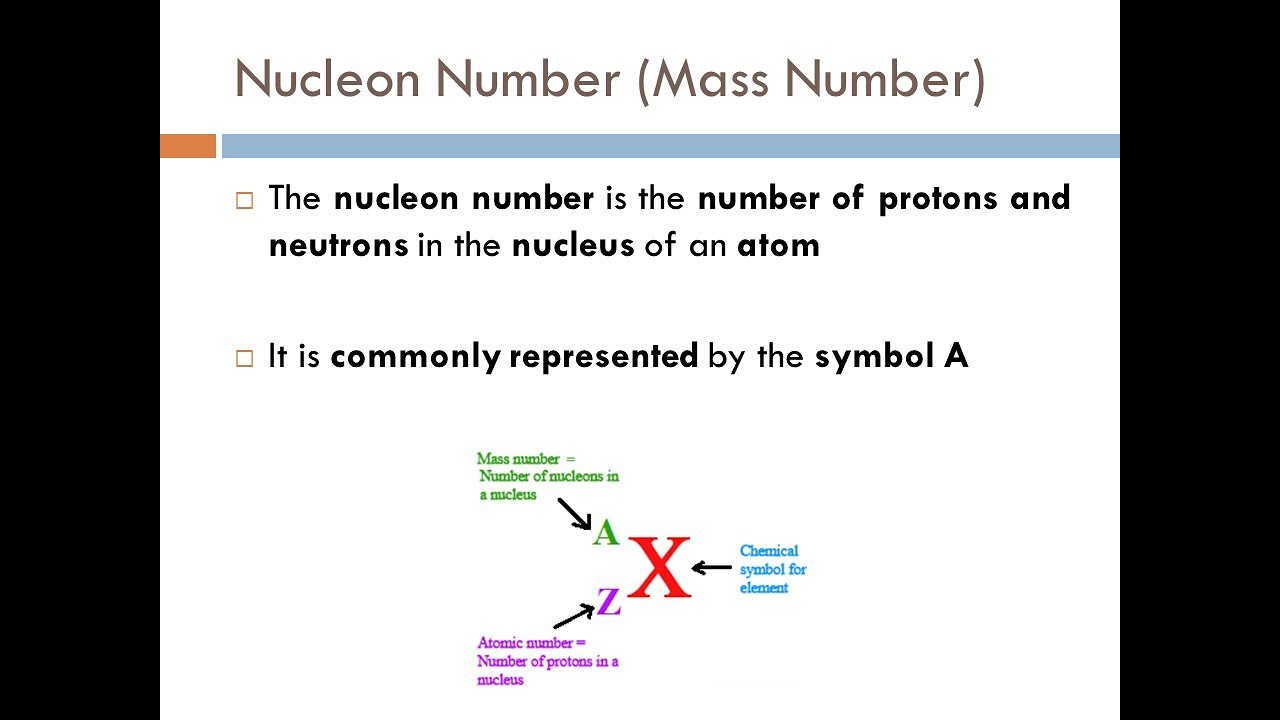
- 🔢 The atomic number refers to the number of protons in the nucleus, while the mass number includes both protons and neutrons.
- 🤖 Nuclide symbols represent atoms with one or two letters (element abbreviation), with the first letter capitalized and the second not.
- 💡 The mass number is indicated at the upper left, distinguishing isotopes of an element that have different neutron counts.
- 🌀 Isotopes are atoms of the same element with varying numbers of neutrons, leading to different masses.
- 🔋 Electron count can differ, leading to ions: a neutral atom has equal numbers of protons and electrons, while ions have an imbalance.
- 📊 The atomic masses on the periodic table are average atomic masses, reflecting the relative abundance of each isotope.
- 🧪 Fractions in atomic masses are due to averaging the masses of all isotopes, accounting for their prevalence in nature.
- 📈 For example, chlorine's average atomic mass isn't 36 because of the prevalence of chlorine-35 over chlorine-37.
- 💌 Professor Dave provides educational content and can be reached via email at ProfessorDaveExplains@gmail.com.
Q & A
What determines the type of element an atom belongs to?
-The number of protons in the nucleus determines the type of element an atom belongs to.
What is the term used for the number of protons in an atom's nucleus?
-The number of protons in an atom's nucleus is referred to as the atomic number.
What contributes to an atom's mass number?
-An atom's mass number is determined by the combined number of protons and neutrons in the nucleus.
Why are electrons typically ignored when calculating the mass of an atom?
-Electrons are much less massive than protons and neutrons, so their mass is negligible when calculating the atom's overall mass.
How is an atom represented in nuclide symbols?
-In nuclide symbols, an atom is represented by one or two letters (capitalized accordingly) that abbreviate the element, with the atomic number (redundantly) and mass number placed in specific positions.
What are atoms of the same element with different numbers of neutrons called?
-Atoms of the same element with different numbers of neutrons are called isotopes.
How is the electrical charge of an atom indicated in its nuclide symbol?
-The electrical charge of an atom is indicated in the upper right corner of the nuclide symbol, with a -1 charge for an extra electron and a +1 charge for a missing electron.
What is the purpose of listing the atomic number next to the element symbol in a nuclide symbol?
-While it is somewhat redundant since the element type implies the number of protons, the atomic number is sometimes listed to explicitly indicate the number of protons in the nucleus.
Why are the atomic masses on the periodic table not whole numbers?
-The atomic masses on the periodic table are average atomic masses, which account for the relative abundance of each isotope of an element, as isotopes have different numbers of neutrons.
How is the average atomic mass for an element with multiple isotopes calculated?
-The average atomic mass is calculated by multiplying each isotope's mass number by a fraction representing its abundance and then summing these values to get a more accurate average mass for all atoms of that element.
What is the significance of the periodic table's arrangement by atomic number?
-The periodic table is arranged by atomic number to organize all elements according to the number of protons in their nuclei, which dictates their chemical properties and behavior.
Outlines
🔬 Introduction to Nuclide Symbols and Atomic Structure
This paragraph introduces the audience to the basics of atomic structure, explaining the roles of protons, neutrons, and electrons. It emphasizes the importance of protons in determining the element's identity and introduces the concept of atomic and mass numbers. The explanation also touches on the representation of atoms using nuclide symbols, including the notation for isotopes and ions, and the significance of these symbols in understanding atomic composition.
Mindmap
Keywords
💡nuclide symbols
💡atomic number
💡mass number
💡protons
💡neutrons
💡electrons
💡isotopes
💡ions
💡periodic table
💡average atomic mass
Highlights
Atoms have a nucleus composed of positively charged protons and neutral neutrons, along with negatively charged electrons orbiting at a distance.
The proton is the particle that determines the element to which an atom belongs.
An element's atomic number corresponds to the number of protons in its nucleus.
The mass number of an atom accounts for both protons and neutrons, each approximately equal to 1 atomic mass unit.
Electrons are much less massive than protons and neutrons, and are often ignored in mass calculations.
A nuclide symbol represents an atom using one or two letters for the element and may include the atomic and mass numbers.
The atomic number is sometimes redundantly included in nuclide symbols, as the element type implies the number of protons.
Isotopes of an element differ in mass due to having different numbers of neutrons.
The mass number is calculated as the sum of protons and neutrons, revealing the number of neutrons by subtracting the atomic number.
An ion is an atom or molecule with an electrical charge due to the loss or gain of electrons.
Neutral atoms have equal numbers of protons and electrons, thus balancing positive and negative charges.
The periodic table arranges all elements by atomic number, from 1 proton to over a hundred.
Average atomic masses on the periodic table account for the relative abundance of each isotope of an element.
Isotopes like chlorine-35 and chlorine-37 differ in the number of neutrons, with average atomic masses reflecting their prevalence.
The average atomic mass is calculated by multiplying each mass number by its fractional abundance and summing these values.
Individual atoms have whole number mass numbers, while the periodic table displays average atomic masses.
The video aims to educate viewers on the fundamentals of nuclide symbols, isotopes, and atomic structure.
Transcripts
Browse More Related Video

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2

What's the Difference between Mass Number and Atomic Mass?

Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry

Lesson 10 - What is Atomic Mass Of An Element? (Chemistry Tutor)
Learning Outcomes (d) and (e) from Atomic Structure - JC H2 Chemistry

Atomic Number, Mass Number, and Net Electric Charge
5.0 / 5 (0 votes)
Thanks for rating: