Organic Chemistry Reactions Summary
TLDRThis video script offers an in-depth exploration of essential organic chemistry reactions, focusing on mechanisms and outcomes of various reactions such as alkene and alkyne transformations, carbocation rearrangements, and the impact of different reagents on reaction products. It covers topics like Markovnikov and anti-Markovnikov addition, oxymercuration-demercuration, and the effects of peroxides on reaction pathways. The script also delves into the nuances of SN1, SN2, E1, and E2 reactions, as well as the reduction of ketones and aldehydes using sodium borohydride and lithium aluminum hydride, providing a comprehensive guide for students preparing for organic chemistry exams.
Takeaways
- 🧪 The major product of butane reacting with hydrobromic acid is an alkene, where the hydrogen attaches to the primary carbon and the bromide ion forms a secondary carbocation for stability.
- 🔁 Hydrobromic acid without peroxides follows Markovnikov's rule, attaching the bromine to the more substituted carbon, while with peroxides, anti-Markovnikov products are formed.
- 🔬 When cyclohexene reacts with bromine in dichloromethane, it undergoes anti-addition, resulting in a mixture of products including an enactment.
- ⏲ The NBS reaction with cyclohexene is a radical reaction that replaces an allylic hydrogen with a bromine atom without affecting the double bond's position.
- 🌞 Under ultraviolet light, bromine in an alkane selectively substitutes tertiary hydrogens due to the stability of tertiary radicals in a radical substitution reaction.
- 🌡 Acid-catalyzed hydration of alkenes results in the formation of an alcohol, with the OH group typically attaching to the most substituted carbon.
- 🔄 Hydroboration-oxidation of alkenes converts them into alcohols without carbocation rearrangements, following anti-Markovnikov's rule and syn stereochemistry.
- 🌀 Oxymercuration-demercuration of alkenes like cyclohexene results in the formation of a tertiary alcohol, with the OH group attaching to the more substituted carbon.
- 💧 Reaction of cyclohexene with mCPBA forms an epoxide, which can further react with water to form a trans-diol through anti-addition.
- 🔌 The reduction of alkynes with hydrogen gas and platinum catalyst converts them completely to alkanes, while sodium metal and liquid ammonia stop at the trans alkene level.
- ⚛️ Hydroboration of terminal alkynes results in the formation of an aldehyde, while with mercury sulfate it leads to the formation of a ketone after tautomerization.
Q & A
What is the major product when butane reacts with hydrobromic acid?
-The major product is an alkene with the hydrogen atom attaching to the primary carbon of the double bond, and the bromide ion being expelled. This occurs because secondary carbocations are more stable than primary carbocations, which would be formed if the hydrogen attached to the secondary carbon.
Why does the hydrogen go to the primary carbon in the reaction of butane with hydrobromic acid?
-The hydrogen goes to the primary carbon to place a positive charge on a secondary carbon, which is more stable than a positive charge on a primary carbon, thus avoiding the formation of an unstable primary carbocation.
What is the result of the bromine atom's position in the reaction of an alkene with hydrobromic acid and peroxides?
-With peroxides, the bromine atom will go to the least substituted carbon (primary carbon) of the double bond, contrary to the Markovnikov regiochemistry that places the bromine atom on the more substituted carbon (tertiary carbon) when no peroxides are used.
What type of addition occurs when cyclohexene reacts with bromine in dichloromethane?
-The reaction proceeds with anti-addition, resulting in one bromine atom on the wedge and the other on the dash, leading to a mixture of two products due to the enantiomeric excess.
How does the reaction of NBS with cyclohexene differ from other reactions discussed in the script?
-The reaction with NBS is a radical reaction, where an allylic hydrogen is replaced with a bromine atom without affecting the position of the double bond, resulting in a single product due to the symmetrical nature of cyclohexene.
What is the major product of the reaction between an alkane and bromine under ultraviolet light?
-The reaction is a radical substitution reaction, where a hydrogen atom, specifically the most substituted tertiary hydrogen, is replaced by a bromine atom due to the stability of the tertiary radical formed.
What is the outcome of the acid-catalyzed hydration of an alkene?
-The reaction produces an alcohol with the OH group typically found on the most substituted carbon. This occurs through a mechanism involving proton abstraction, carbocation rearrangement, and combination with water to form an oxonium species, which then loses a proton to form the alcohol.
What is the difference between the reactions of alkynes with hydrogen gas and platinum catalyst versus sodium metal and liquid ammonia?
-With hydrogen gas and platinum catalyst, the alkyne is fully reduced to an alkane, while with sodium metal and liquid ammonia, the reaction stops at the trans alkene level, forming a cis alkene with Lindlar's catalyst.
What is the major product of the hydroboration-oxidation reaction of an alkene?
-The reaction converts an alkene to an alcohol without carbocation rearrangements, with the OH group being placed on the least substituted carbon atom of the double bond, following anti-Markovnikov regiochemistry.
What is the result of the oxymercuration-demercuration reaction of an alkene?
-The reaction converts an alkene into a tertiary alcohol, with the OH group placed on the more substituted carbon atom of the double bond, following Markovnikov's rule.
What happens when an alkene reacts with mCPBA?
-The alkene is converted into an epoxide, which can then react with water in the presence of acid to form a trans-diol through an anti-addition mechanism.
What is the major product of the reaction between an alkyne and sodium amide followed by methyl bromide?
-The reaction proceeds through an SN2 mechanism, where the acetylide ion, formed by deprotonation of the alkyne by sodium amide, reacts with methyl bromide, resulting in the formation of a new carbon-carbon bond.
What is the difference between the reactions of 2-bromobutane with potassium iodide in acetone versus water?
-In acetone, an SN2 reaction occurs, leading to inversion of configuration and formation of an alkyl iodide. In water, an SN1 reaction is favored, leading to a racemic mixture of products due to the possibility of attack from the front or back on the carbocation intermediate.
What is the major product of the E1 elimination reaction of an alcohol with sulfuric acid upon heating?
-The reaction proceeds through an E1 mechanism, where the alcohol is protonated, the hydroxyl group leaves as water, and a carbocation is formed. The base then abstracts a proton to form a more stable alkene, specifically a tetra-substituted alkene, which is the most stable product.
What is the outcome of reducing a ketone with sodium borohydride?
-Sodium borohydride reduces a ketone to an alcohol by adding a hydride ion to the carbonyl carbon, forming an alkoxide ion, which then reacts with water to form the alcohol.
What happens when a ketone is reacted with the Grignard reagent followed by acidification?
-The Grignard reagent, being a nucleophile, adds a methyl group to the carbonyl carbon, forming an alkoxide ion, which upon acidification with water forms a tertiary alcohol.
Outlines
🔍 Organic Chemistry Reactions Overview
This paragraph introduces a variety of common organic chemistry reactions essential for final exam preparation. It begins with the reaction of butane with hydrobromic acid, detailing the formation of an alkene and the subsequent creation of a carbocation. The discussion highlights the stability of secondary carbocations over primary ones and the production of a racemic mixture due to the bromide ion's attack. The paragraph further compares reactions with and without peroxides, emphasizing Markovnikov's regiochemistry and the different products formed. It also covers reactions involving cyclohexene, including anti-addition with bromine and NBS, and the selective substitution of tertiary hydrogens in alkanes under UV light, which is a radical reaction.
🧪 Detailed Mechanisms of Organic Reactions
The second paragraph delves into the mechanisms behind various organic reactions, starting with acid-catalyzed hydration of alkenes, which results in the formation of alcohols. It explains the process involving the abstraction of a proton by the alkene, the formation of a more stable tertiary carbocation through a hydride shift, and the final combination with water to form an oxonium species leading to an alcohol. The paragraph also discusses the reduction of cyclohexene with hydrogen gas using a metal catalyst, the use of deuterium with palladium catalyst for stereospecific reduction, and the hydroboration-oxidation sequence that converts alkenes to alcohols without carbocation rearrangements. It concludes with an invitation to view a more detailed review of these mechanisms in the video's playlist.
🌀 Reactions of Alkenes and Alkynes with Different Reagents
This paragraph explores the reactions of alkenes and alkynes with various reagents, focusing on the products formed under different conditions. It discusses the conversion of cyclohexene to an alcohol through oxymercuration-demercuration and the formation of epoxides with mCPBA, followed by the opening of the epoxide ring to form diols. The paragraph also covers the conversion of alkenes into cis- or trans-diols and the reaction of alkynes with hydrogen gas and different catalysts, resulting in alkanes, alkenes, or enols that tautomerize into aldehydes or ketones. It concludes with the reaction of terminal alkynes with sodium amide and alkyl halides, which leads to the formation of new carbon-carbon bonds.
🔬 Substitution and Elimination Reactions in Organic Chemistry
The fourth paragraph examines substitution and elimination reactions in organic chemistry. It starts with the reaction of 2-butyne with hydrogen gas and a platinum catalyst to form an alkane and compares it with reactions using sodium metal and liquid ammonia or Lindlar's catalyst, which yield different alkene products. The paragraph then describes the hydroboration of terminal alkynes to form aldehydes and the reaction of acetylene with mercury sulfate to produce ketones. It also discusses the use of sodium amide and methyl bromide to create carbon-carbon bonds and the multi-step reaction of acetylene to form a specific alkene. The paragraph concludes with the SN2 reaction of 2-bromobutane with potassium iodide and acetone, resulting in inversion of configuration.
📚 SN1, E1, and E2 Reaction Mechanisms in Organic Chemistry
This paragraph explains the SN1, E1, and E2 reaction mechanisms in organic chemistry. It discusses the SN1 reaction involving a secondary alkyl halide and water, leading to a racemic mixture due to the attack of water from the front and back on the carbocation. The E1 reaction is also explored, where water acts as a base to form an alkene, with the major product being trans-2-butene. The paragraph further explains the E2 reaction, which occurs with strong bases and does not involve carbocation intermediates, leading to the most stable alkene, the Zaitsev product. It also touches on the effect of sterically hindered bases and substrates on the reaction outcome, which can lead to the Hofmann product instead.
🧪 Oxidation and Reduction Reactions in Organic Chemistry
The sixth paragraph focuses on oxidation and reduction reactions in organic chemistry. It starts with the oxidation of primary alcohols to aldehydes using PCC and to carboxylic acids with chromic acid. The oxidation of secondary alcohols to ketones is also discussed, regardless of the oxidizing agent's strength. Tertiary alcohols are noted to be resistant to oxidation. The paragraph then moves on to reduction reactions, where sodium borohydride and lithium aluminum hydride are used to reduce aldehydes, ketones, and acid chlorides to alcohols, with the latter also capable of reducing esters and carboxylic acids. The mechanisms for these reductions are briefly outlined, highlighting the role of hydride ions in breaking carbonyl bonds and forming new C-H bonds.
🌐 The Role of Grignard Reagent in Organic Reductions
The final paragraph discusses the use of the Grignard reagent in organic reductions. It explains how the reagent, formed from methyl magnesium bromide, can reduce ketones to alcohols by adding a methyl group instead of a hydride. The nucleophilic nature of the carbon in the Grignard reagent allows it to attack the electrophilic carbon of the carbonyl group, forming an alkoxide ion that subsequently reacts with water to form a tertiary alcohol. The paragraph emphasizes the versatility of the Grignard reagent in reducing various functional groups to alcohols and concludes the video with a summary of the covered topics.
Mindmap
Keywords
💡Organic Chemistry Reactions
💡Nucleophile
💡Electrophile
💡Carbocation
💡Markovnikov Regiochemistry
💡Anti-Addition
💡Radical Reaction
💡Acid-Catalyzed Hydration
💡Hydroboration Oxidation
💡Oxymercuration Demercuration
💡Epoxide
💡Alkyne Reduction
💡Hydroboration
💡SN2 Reaction
💡E1 Reaction
💡E2 Reaction
💡Oxidation Reactions
💡Reduction Reactions
Highlights
Introduction to common organic chemistry reactions for final exam preparation.
Explanation of the major product formation when butane reacts with hydrobromic acid, focusing on nucleophilic and electrophilic reactions.
Stability preference for secondary carbocations over primary in alkene reactions.
Formation of a racemic mixture in the reaction of alkene with hydrobromic acid due to bromide ion attack.
Markovnikov regiochemistry in alkene reactions with hydrobromic acid without peroxides.
Anti-Markovnikov product formation with peroxides in alkene reactions with hydrobromic acid.
Anti-addition in the reaction of cyclohexene with bromine in dichloromethane.
Radical reaction mechanism in NBS reaction with cyclohexene, leading to allylic bromination.
Free radical substitution reaction of alkane with bromine under UV light, favoring tertiary hydrogen replacement.
Acid-catalyzed hydration of alkenes leading to alcohol formation with carbocation rearrangement.
Hydrogenation of cyclohexene to cycloalkane using metal catalysts.
Deuterium addition to cyclohexene with palladium catalyst illustrating syn stereochemistry.
Hydroboration-oxidation of alkenes to produce alcohols without carbocation rearrangements.
Oxymercuration-demercuration reaction sequence converting alkenes into tertiary alcohols.
Conversion of alkenes into epoxides using mCPBA and subsequent opening to form trans-diols.
Syn stereochemistry in the conversion of alkenes to cis-diols using osmium tetroxide and sodium bisulfite.
Selective reduction of alkynes to cis-alkenes or trans-alkenes using different catalysts and reagents.
Hydroboration of terminal alkynes leading to the formation of aldehydes.
Reaction of terminal alkynes with mercury sulfate yielding ketones.
Synthesis of alkene chains via sequential reactions of acetylene with NaNH2 and various alkyl halides.
SN2 reaction mechanism with secondary alkyl halides in polar aprotic solvents leading to inversion of configuration.
SN1 and E1 reaction mechanisms with secondary alkyl halides in protic solvents producing a racemic mixture.
E2 reaction mechanism with strong bases and unhindered substrates favoring the most stable alkene (Zaitsev's rule).
Dehydration of secondary alcohols to form tetra-substituted alkenes via E1 mechanism.
Conversion of alcohols to alkyl halides via SN1 mechanism with hydrobromic acid resulting in racemic mixtures.
Oxidation of primary alcohols to aldehydes using PCC and to carboxylic acids with chromic acid.
Reduction of ketones to alcohols using sodium borohydride and lithium aluminum hydride.
Grignard reagent's ability to reduce ketones to alcohols by adding a methyl group.
Transcripts
Browse More Related Video

12.3 Synthesis of Alcohols | Organic Chemistry

Aldehydes and Ketones
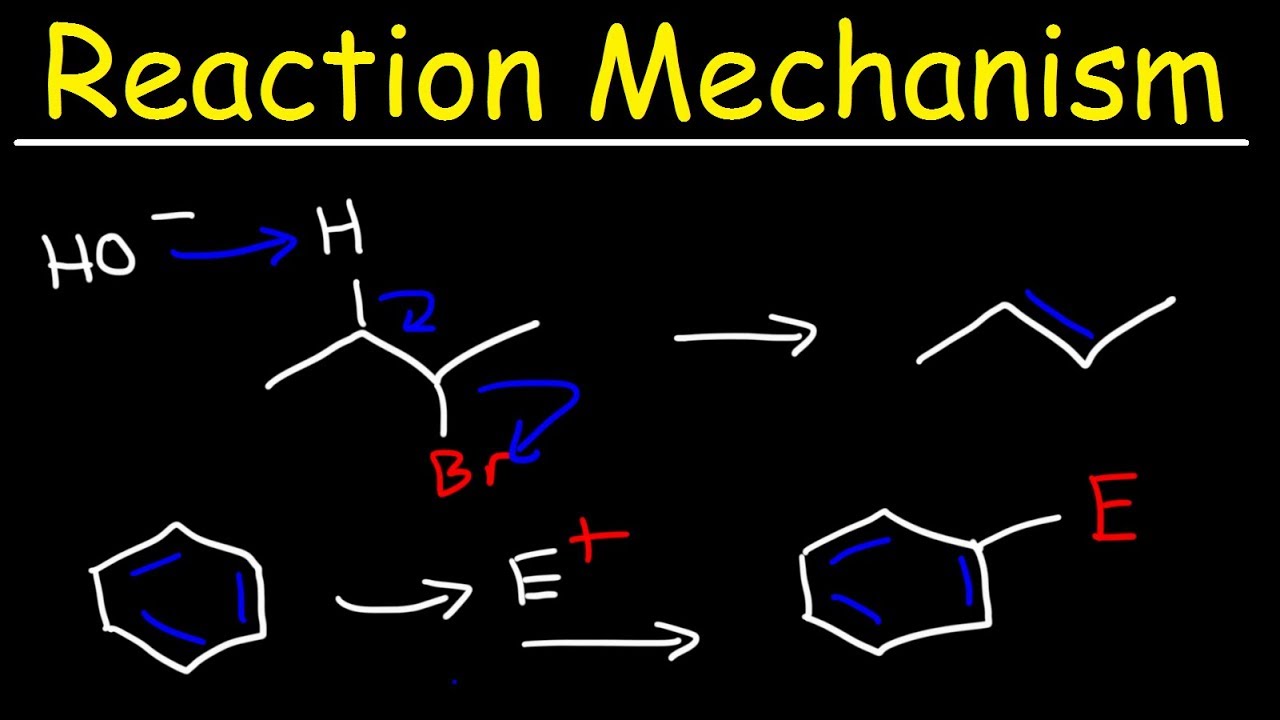
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement

SN1 SN2 E1 E2 Reaction Mechanism - Test Review

Organic Chemistry 1 Exam 2 Review Questions

8.3 Acid Catalyzed Hydration, Oxymercuration Demercuration, and Hydroboration Oxidation | OChemistry
5.0 / 5 (0 votes)
Thanks for rating: