Ep21 The glassy state and the glass transition - UCSD NANO 134 Darren Lipomi
TLDRThis script delves into the science of the glass transition (Tg) and its impact on the mechanical properties of materials. It explains how Tg affects stress-strain behavior, distinguishing between glassy and rubbery states. The discussion covers the kinetic trapping of the glassy state, sub-Tg relaxation mechanisms contributing to polymer toughness, and how structural factors influence Tg. The script also touches on the time-dependent mechanical behavior of viscoelastic solids and introduces the concept of strain, stress, and Poisson's ratio in the context of polymer deformation.
Takeaways
- 📚 The lecture discusses the glass transition (Tg) and its impact on the mechanical properties of materials, focusing on amorphous samples and amorphous domains in semicrystalline polymers.
- 🔍 The glassy state is described as kinetically trapped, with the crystalline state being more favorable due to better van der Waals interactions between chains.
- 🍝 The glassy state is likened to 'frozen spaghetti', where strain is accommodated through local bending and unbending of bonds rather than chain sliding.
- 💪 Sub-Tg relaxation mechanisms contribute to the toughness of polymers, allowing them to absorb more energy before fracture compared to ceramics, due to local bond rotations like the 'crankshaft mechanism'.
- 🔑 The difference between sub-Tg relaxation and random confirmation movement is highlighted, with the former being triggered by mechanical energy concentration at coent bonds.
- 🌡️ The glass transition is characterized by changes in mechanical properties, specific volume, modulus, heat capacity, dielectric constant, and refractive index as the temperature crosses Tg.
- 🔄 The absence of a discontinuity in entropy or volume at Tg distinguishes it from first-order phase transitions like melting, where there is a latent heat.
- ⏱️ Time-dependent mechanical behavior is introduced, explaining how materials like chewing gum show immediate response to strain but change over time as molecules realign.
- 🔑 Factors affecting Tg include polymer backbone flexibility, steric effects of pendant groups, and configurational isomerism, which influence packing efficiency and intermolecular forces.
- 🔍 The concept of free volume is introduced as a determinant of Tg, with more free volume leading to a lower Tg due to reduced energy needed for chain sliding.
- 🛠️ Methods to control free volume, and thus Tg, include polydispersity, low molecular weight, plasticizers, branching, and residual solvents, all of which can increase free volume.
Q & A
What is the glass transition temperature (Tg) and why is it significant?
-The glass transition temperature (Tg) is the temperature at which a material transitions from a glassy state to a rubbery or viscous liquid state. It is significant because it marks a change in the mechanical properties of a material, such as a drastic increase in toughness and a decrease in modulus, which are important for material applications and performance.
How does the glassy state accommodate strain?
-In the glassy state, materials accommodate strain through local bending and unbending of bonds, as there is not enough thermal energy for the chains to slide past each other. Large stresses are dissipated by breaking covalent bonds, leading to fracture.
What are sub-Tg relaxation mechanisms and why are they important?
-Sub-Tg relaxation mechanisms are local rotations of bonds that can dissipate energy without deforming the sample as a whole. They are important because they contribute to the toughness of a polymer sample, allowing it to absorb more energy before fracture compared to ceramics.
Can you explain the analogy of a frozen rubber hose wound into a helix to describe the mechanical properties near Tg?
-The analogy of a frozen rubber hose wound into a helix is used to describe how materials behave near their Tg. Below Tg, the material is stiff and stores mechanical energy like a spring. At Tg, the material starts to dissipate energy and behaves like a weaker spring with more damping. Above Tg, the material is rubbery and its springiness is controlled by molecular mechanisms, with low damping and time dependence.
What is the difference between the mechanical behavior of amorphous and semicrystalline polymers?
-Amorphous polymers can have a Tg where they transition from a glassy to a rubbery state. Semicrystalline polymers, however, have crystalline domains that act like cross-links, maintaining the material's structure even above Tg. This results in different mechanical properties and behaviors depending on the temperature relative to Tg.
How does the presence of free volume affect the Tg of a polymer?
-The presence of free volume in a polymer can lower the Tg because it takes less energy for the polymer chains to slide past each other when there is more space available. Factors that increase free volume, such as polydispersity, low molecular weight, plasticizers, and branching, can all lead to a lower Tg.
What is the role of specific volume in the context of Tg?
-Specific volume, which is the inverse of density, changes at Tg. As the temperature increases towards Tg, the specific volume increases, indicating a decrease in density. This change in density is associated with the change in the material's mechanical properties.
How does the modulus of a material change as it approaches Tg?
-The modulus of a material, which is a measure of its stiffness, decreases as the material approaches Tg. This is due to the increased molecular motion and the ability of polymer chains to slide past each other, leading to a more flexible and less rigid material.
What is the significance of the heat capacity change at Tg?
-The heat capacity of a material in its rubbery state above Tg is greater than in its glassy state below Tg. This indicates that more energy is required to increase the temperature of the material in the rubbery state, reflecting changes in molecular motion and structure.
Can you provide an example of how mechanical energy can induce chemical bond breakage in a polymer?
-An example is chewing gum, where prolonged mechanical energy from chewing can eventually break chemical bonds in the gum. This process, known as mechanochemistry, demonstrates that mechanical forces can accumulate over time and lead to chemical changes in polymers.
Outlines
📚 Introduction to Glass Transition and Mechanical Properties
The script begins with a discussion on the glass transition (Tg) and its impact on the mechanical properties of materials. It explains that materials exhibit different behaviors below and above Tg, affecting their stress-strain characteristics. The glassy state is described as kinetically trapped, with local bending and unbending of bonds as a means of strain accommodation. Sub-Tg relaxation mechanisms contribute to the toughness of polymers, which is the energy absorbed before fracture. The talk also touches on the difference between amorphous and crystalline regions in semicrystalline samples, emphasizing the importance of Tg in understanding material behavior.
🔄 Sub-Tg Relaxation and Polymer Chain Dynamics
This paragraph delves into the sub-Tg relaxation mechanisms that allow polymer chains to dissipate energy without deforming the entire sample. It uses the analogy of a 'crankshaft mechanism' to describe bond rotations in polymers like polyethylene, which contribute to their toughness. The discussion also covers how these mechanisms are not present in inorganic glasses due to the difference in atomic bonding. The paragraph further explores the conditions under which these relaxation mechanisms occur, including the influence of mechanical force and the transition to more energetically favorable states.
🌡 Understanding the Glass Transition through Analogies
The script uses an analogy of a semicrystalline polymer to explain the glass transition. It describes how, as temperature increases above Tg, the material transitions from a stiff, spring-like state to a weaker, more damping state due to the increased mobility of polymer chains. The analogy of a rubber hose wound into a helix is used to illustrate the mechanical properties of the material at different temperatures relative to Tg and Tm (melting temperature). The paragraph highlights the changes in mechanical properties, such as damping and time dependence, as the material warms through the Tg.
🕰 Time-dependent Mechanical Behavior in Viscoelastic Solids
This section discusses the time-dependent mechanical behavior of viscoelastic solids, contrasting it with the immediate response seen in ideal solids. It uses the example of chewing gum to illustrate how materials can exhibit different mechanical properties over time, with the restoring force weakening as molecules realign. The paragraph also touches on the concept of mechanochemistry, where mechanical energy can break chemical bonds over time, as demonstrated by the degradation of chewing gum.
🔍 Characteristics and Effects of the Glass Transition
The script provides a detailed look at the glass transition, describing it as a second-order phase transition with significant changes in mechanical properties, specific volume, modulus, heat capacity, dielectric constant, and refractive index. It emphasizes the absence of a discontinuity in entropy or volume at Tg, unlike first-order phase transitions, and discusses the technological importance of Tg. The paragraph also explores factors that influence Tg, such as polymer flexibility and chemical structure.
🔬 Structural Factors Influencing Glass Transition Temperature
This paragraph examines the structural characteristics of polymers that affect their Tg, such as the flexibility of the polymer backbone and the presence of bulky side groups. It provides examples of different polymers, like PDMS, polyethylene, and PEO, and their respective Tg values. The discussion also covers the impact of steric effects, configurational isomerism, and the importance of efficient packing on Tg, with examples of polymers like PPO, PP, and PMMA to illustrate these concepts.
🌟 The Role of Free Volume and Molecular Packing in Tg
The script explains the concept of free volume and how it relates to the Tg of a polymer. It describes the van der Waals volume, packing volume, and the role of molecular shape and size in determining free volume. The paragraph discusses how factors like polydispersity, plasticizers, branching, and residual solvents can increase free volume, thereby lowering Tg. It also touches on the idea that more free volume results in a lower Tg due to reduced intermolecular forces.
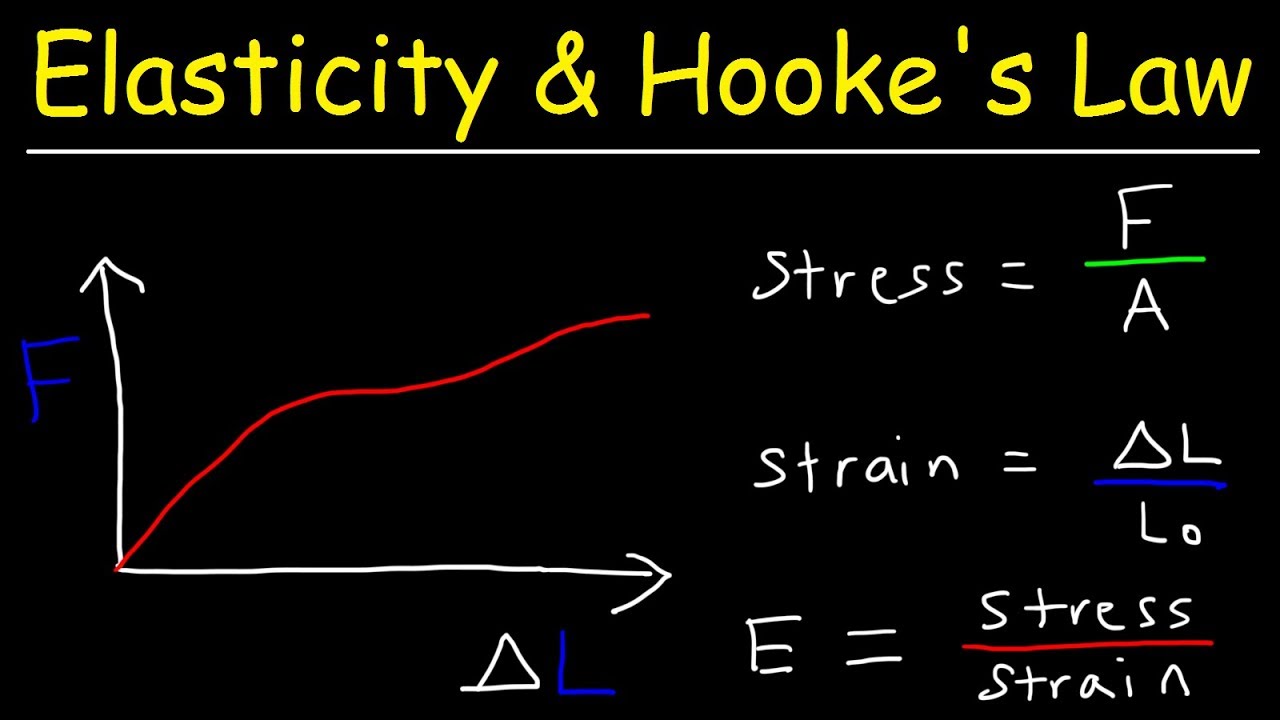
📏 Fundamentals of Stress and Strain in Polymer Mechanics
The final paragraph introduces the basic concepts of stress and strain in the context of polymer mechanics. It defines strain as the change in length relative to the original length and stress as the force per unit area causing deformation. The paragraph also introduces the Poisson's ratio, which describes the transverse contraction of a material under axial strain. The discussion sets the stage for further exploration of stress-strain curves and viscoelasticity in subsequent lessons.
Mindmap
Keywords
💡Glass Transition
💡Mechanical Properties
💡Stress-Strain Behavior
💡Sub-Tg Relaxation Mechanisms
💡Toughness
💡Amorphous and Semicrystalline Samples
💡Vander Waals Interactions
💡Plasticizers
💡Free Volume
💡Stress and Strain
💡Pon Ratio
Highlights
Discussion of the glass transition and its impact on the mechanical properties of materials.
Exploration of how the glassy, crystalline, and amorphous states influence stress-strain behavior relative to Tg (glass transition temperature) and Tm (melting temperature).
Introduction to the concept of amorphous samples and semicrystalline domains, emphasizing the irrelevance of Tg for crystalline regions.
Explanation of the kinetically trapped glassy state and its preference for crystalline state due to better van der Waals interactions.
Mechanism of strain accommodation in the glassy state through local bending and unbending of bonds, highlighting the role of sub-Tg relaxation mechanisms in polymer toughness.
Comparison of toughness in glassy polymers to ceramics, attributing the higher toughness to sub-Tg relaxation mechanisms.
Description of the crankshaft mechanism in polymers, illustrating energy dissipation without overall deformation.
Differentiation between the energy dissipation mechanisms in polymers and inorganic glasses, emphasizing the one-dimensionality of polymer chains.
Analogy of the glass transition in semicrystalline samples using a frozen rubber hose to explain mechanical properties at different temperatures.
Discussion on the time-dependent mechanical behavior of viscoelastic solids and its comparison to immediate response in ideal solids.
Technical insight into the decomposition of chewing gum through prolonged mechanical energy application, leading to bond cleavage.
Clarification of Tg as a second-order phase transition with significant changes in mechanical properties but without a discontinuity in entropy or volume.
Analysis of structural characteristics affecting Tg, such as polymer backbone flexibility and steric effects of pendant groups.
Impact of configurational isomerism on Tg, exemplified by the difference in Tg between cis and trans forms of polybutene.
Explanation of how free volume influences Tg, with more free volume leading to a lower Tg due to reduced intermolecular forces.
Methods to control free volume, including polydispersity, low molecular weight, plasticizers, and branching, and their effects on Tg.
Introduction to stress and strain concepts, essential for understanding mechanical properties in relation to Tg.
Definition and significance of the Poisson's ratio in the context of mechanical deformation.
Overview of mechanical meta materials with unusual Poisson's ratios, including materials that expand in the transverse dimension under axial load.
Transcripts
Browse More Related Video

Ep22 Mechanical properties of polymers & viscoelastic models NANO 134 UCSD Darren Lipomi
Elasticity & Hooke's Law - Intro to Young's Modulus, Stress & Strain, Elastic & Proportional Limit

Ep17 Chain models and DSC - NANO 134 Darren Lipomi UCSD

Fatigue Failure | Engineering Approach

Ep20 Block copolymers & Liquid crystals NANO 134 UCSD Darren Lipomi

Ep13 Cloud point and phase diagrams - UC San Diego - NANO 134 Darren Lipomi
5.0 / 5 (0 votes)
Thanks for rating: