Phases and components
TLDRThis script delves into the fundamental concepts of phase diagrams, defining 'phase' as a chemically homogeneous and mechanically separable part of a system, with different crystal structures considered distinct phases. It illustrates the idea using iron's various solid phases. The script also explains 'component' as an independent chemical species, essential for system composition. Examples provided include water as a single-component system and mild steel as a two-component, two-phase system. The lecture hints at the Gibbs phase rule and the significance of binary phase diagrams in material science.
Takeaways
- 🔬 A phase is defined as a chemically homogeneous, physically distinct, and mechanically separable part of a system.
- 🌡️ The traditional phases of matter include solid, liquid, and gas, with solid phases being differentiated by their crystal structures.
- 📚 Different solid phases, such as the alpha and gamma phases of iron, are represented by Greek letters in phase diagrams.
- 🧪 A component is an independent chemical species, which can be a pure element or a compound, and determines the composition of a system.
- 🔉 In a system like water, the fixed ratio of hydrogen to oxygen means it is considered a single component, despite being a compound.
- 🧊 An example of a single component, two phase system is ice floating in water, both composed of H2O but in different phases.
- 🧂 Brine is a two component system where the ratio of sodium chloride to water can vary, but not the ratio within the compounds themselves.
- 🛠️ Mild steel is a two component, two phase system with iron and carbon as components and alpha phase iron and Fe3C as phases.
- 📈 The Gibbs phase rule, which relates the number of components, phases, and degrees of freedom, will be discussed later in the course.
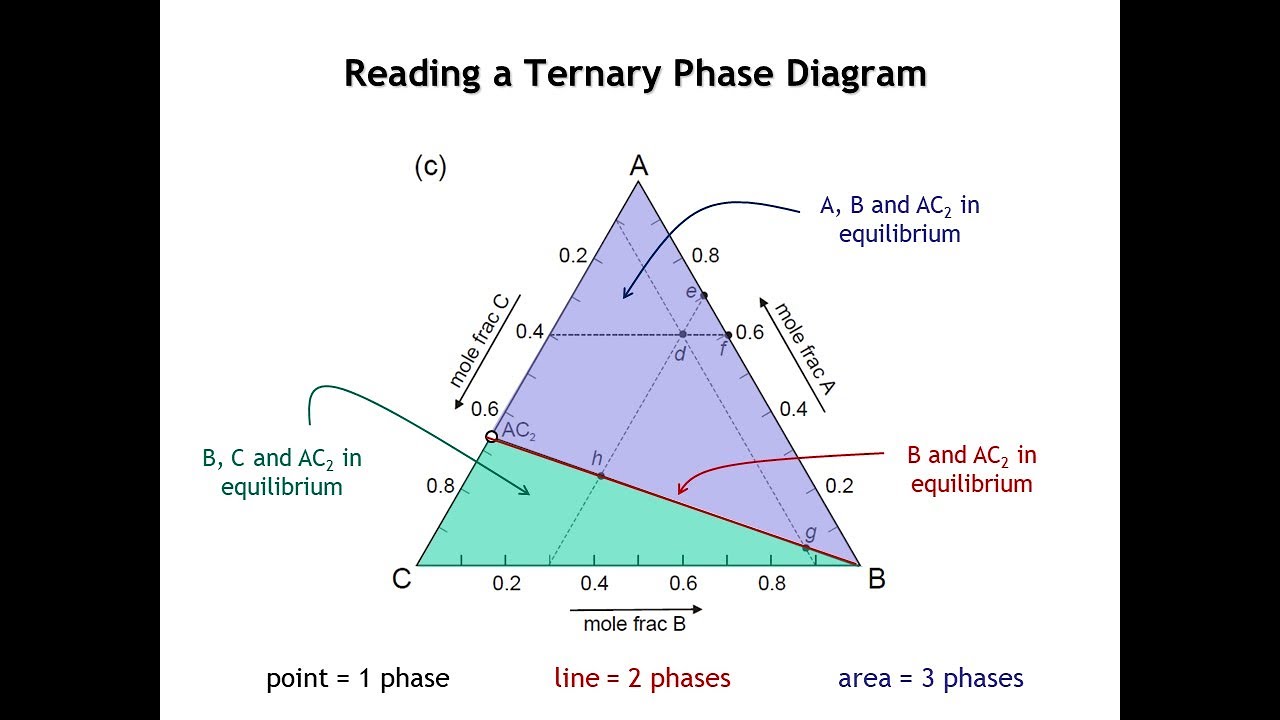
- 📊 Phase diagrams can be classified as unary (single component), binary (two components), ternary (three components), and so on, with the course focusing mainly on binary diagrams.
- 🔑 The script emphasizes the importance of understanding phases and components for the study of phase diagrams in materials science and engineering.
Q & A
What is the traditional definition of a phase in the context of phase diagrams?
-A phase is defined as a chemically homogeneous, physically distinct, and mechanically separable part of a system.
What are the three primary phases of matter that can be represented in a phase diagram?
-The three primary phases of matter that can be represented in a phase diagram are liquid, solid, and gas.
Why are different crystal structures of the same material considered as different solid phases?
-Different crystal structures of the same material are considered as different solid phases because they have distinct physical properties, even though they share the same chemical composition.
How are solid phases in phase diagrams traditionally denoted?
-Solid phases in phase diagrams are traditionally denoted by Greek letters.
What is an example of a material with different solid phases based on its crystal structure?
-Iron is an example of a material with different solid phases. At room temperature, it has a body-centered cubic (BCC) structure, known as the alpha phase, and at high temperatures, it has a face-centered cubic (FCC) structure, known as the gamma phase.
What is the definition of a component in the context of phase diagrams?
-A component is defined as the independent chemical species, which can be a pure element or a compound, in terms of which the composition of a system is specified.
How does the composition of water relate to its status as a single component in phase diagrams?
-Water is considered a single component in phase diagrams because the ratio of hydrogen to oxygen is fixed in H2O, and this composition cannot be varied.
What is an example of a system that is a single component but a two-phase system?
-An example of a single component but a two-phase system is ice floating in water. Both water and ice are composed of H2O, but they represent different phases (liquid and solid).
How does the composition of brine (a solution of sodium chloride in water) differ from that of water in terms of components?
-Brine is a two-component system because the ratio of sodium chloride to water can be varied, unlike in water where the ratio of hydrogen to oxygen is fixed.
What are the components of mild steel, and how many phases are present at room temperature?
-The components of mild steel are iron and carbon. At room temperature, mild steel typically has two phases: the alpha phase (ferrite) and a compound phase, Fe3C (cementite).
What are the different types of phase diagrams based on the number of components involved?
-Phase diagrams can be classified based on the number of components involved: unary diagrams for single components, binary diagrams for two components, ternary diagrams for three components, and so on, with higher-order diagrams for four or more components.
Outlines
🔬 Introduction to Phases and Components in Phase Diagrams
The script introduces the concept of phase diagrams, emphasizing the need to define 'phases' and 'components' for proper discussion. It explains that a phase is a chemically homogeneous, physically distinct, and mechanically separable part of a system. The traditional definition is used, and the script clarifies that different crystal structures, such as the alpha and gamma phases of iron, are considered different phases. The script also introduces the concept of components, which are the independent chemical species that can be elements or compounds, and their role in defining the composition of a system.
🌡 Understanding Components and Phases in Different Systems
This paragraph delves deeper into the definition of components, describing them as independent chemical species that can be pure elements or compounds. It uses the copper-nickel system as an example to illustrate how the composition is specified by the proportion of these components. The script also discusses water as a single-component system, where the ratio of hydrogen to oxygen is fixed, and contrasts it with a two-phase system like ice and water, which still consists of a single component. The concept is further explored with brine, a solution of sodium chloride in water, which is a two-component system where the ratio of sodium chloride to water can vary, and mild steel, which contains iron and carbon as components.
📈 Types of Phase Diagrams and the Significance of Components and Phases
The final paragraph discusses the different types of phase diagrams based on the number of components involved, such as unary, binary, ternary, and higher-order diagrams. It highlights the focus of the course on binary phase diagrams and introduces the concept of the Gibbs phase rule, which will be discussed later. The paragraph also touches on the relationship between the number of components, phases, and degrees of freedom, setting the stage for further exploration of these concepts in the course.
Mindmap
Keywords
💡Phase
💡Component
💡Phase Diagram
💡Solid Phase
💡Gibbs Phase Rule
💡Unary Diagram
💡Binary Diagram
💡Ternary Diagram
💡Degrees of Freedom
💡Crystal Structure
💡Engineering Alloy
Highlights
Introduction to the discussion on phase diagrams without a proper definition of phases and components.
Definition of a phase as a chemically homogeneous, physically distinct, and mechanically separable part of a system.
Clarification that solid phases can be more than one, with different crystal structures considered as different solid phases.
Traditional representation of solid phases in phase diagrams using Greek letters.
Example of different crystal structures of iron, such as body-centered cubic (alpha phase) and cubic close-packed (gamma phase).
Emphasis on the chemical composition and physical state being identical in different solid phases of pure elements like iron.
Definition of a component as an independent chemical species, which can be a pure element or a compound.
Explanation of how the composition of a system is specified by the components, using the copper-nickel system as an example.
Illustration of water as a single component system, with a fixed ratio of hydrogen and oxygen.
Description of a single component, two-phase system, such as water and ice, both composed of H2O.
Introduction of brine as a two-component system with variable ratios of sodium chloride to water.
Differentiation between the number of components and the number of phases in a system, using mild steel as an example.
Mention of the Gibbs phase rule and its relation to the number of components, phases, and degrees of freedom.
Classification of phase diagrams based on the number of components, such as unary, binary, ternary, and higher order diagrams.
Focus on binary phase diagrams for two-component systems, which will be the main subject of the course.
Conclusion of the video with a summary of the importance of understanding phases and components in phase diagrams.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: