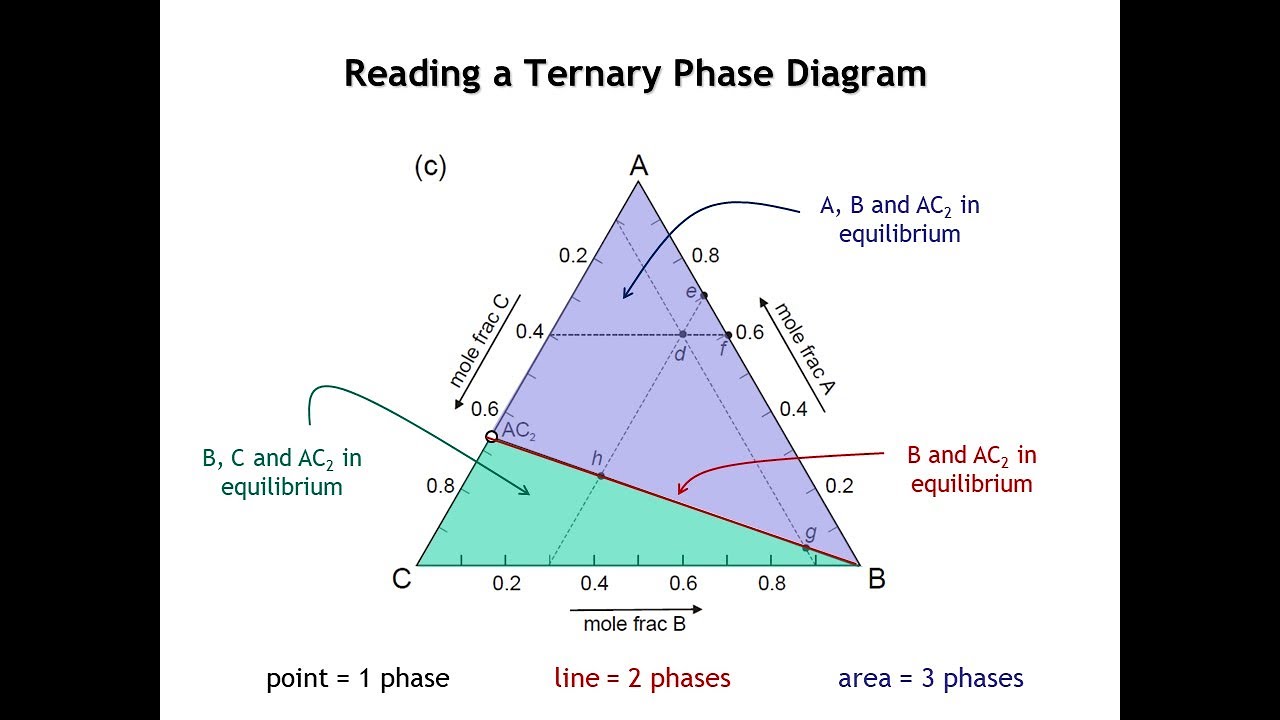
Phase diagrams: Introduction
TLDRThe video script delves into the fundamentals of phase diagrams, a crucial concept in materials science, using copper-nickel alloys as an illustrative example. It explains the creation of phase diagrams by plotting temperature against composition, highlighting key points like melting points and the formation of solid and liquid phases. The script also introduces the solidus and liquidus boundaries, and the concept of a substitution solid solution, governed by Hume-Rothery rules. It concludes by defining an equilibrium phase diagram as a graphical representation of phase equilibria in a space of relevant thermodynamic variables.
Takeaways
- 📚 The topic of the script is Phase Diagrams, an important subject in mechanical science and material science courses.
- 🔍 The script introduces phase diagrams with the example of a copper-nickel alloy, highlighting the importance of understanding the phase behavior in alloy systems.
- 🌡️ The melting points of copper and nickel are given as 1085°C and 1453°C, respectively, setting the stage for understanding phase behavior as a function of temperature.
- 📊 A basic one-dimensional phase diagram is created for copper and nickel, showing the transition from solid to liquid at their respective melting points.
- 🤔 The script discusses the concept of linear interpolation to estimate the melting point of an alloy, such as a 50-50 copper-nickel alloy, which is assumed to be the average of the two metals' melting points.
- 🧪 The actual behavior of the alloy upon melting is found to be different from the linear interpolation prediction, with melting starting at a lower temperature (TS) and completing at a higher temperature (TL).
- 🔨 The script emphasizes the importance of experimental observation in phase diagrams, as it can reveal non-linear behavior and the presence of a melting range rather than a single temperature.
- 📉 The phase diagram for the copper-nickel system is explained with the introduction of the liquidus and solidus boundaries, which define the regions of stability for liquid and solid phases, respectively.
- 🔑 The concept of a substitution solid solution is introduced, explaining how copper and nickel, being of similar size and crystal structure, can form a continuous solid solution.
- 📈 The phase diagram is further elaborated to include the Greek letter 'alpha' to represent the solid solution phase, and the coexistence of liquid and solid phases in the region between the liquidus and solidus lines.
- 🏷️ The script concludes with a definition of an equilibrium phase diagram, emphasizing its role in indicating the phases in equilibrium at different temperatures and compositions.
Q & A
What is a phase diagram in the context of material science?
-A phase diagram is a graphical representation of the conditions under which different phases of a material coexist in equilibrium. It typically plots the variables of temperature, pressure, and composition to show the regions where each phase is stable.
Why is the copper-nickel alloy system used as an example in the script?
-The copper-nickel alloy system is used as an example because it illustrates the basic concepts of phase diagrams, such as the melting points of pure components and how they change when combined to form an alloy.
What is the melting point of copper and how is it represented on a phase diagram?
-The melting point of copper is 1085 degrees Celsius. On a phase diagram, it is represented as a point on the temperature axis where the solid phase of copper coexists with its liquid phase.
What is the melting point of nickel and how does it compare to that of copper?
-The melting point of nickel is 1453 degrees Celsius, which is higher than that of copper. This difference is crucial when considering the phase behavior of the copper-nickel alloy system.
What is the concept of 'linear interpolation' as mentioned in the script?
-Linear interpolation is an assumption that the property of interest (like melting point) varies linearly between the values of the pure components as the composition changes. In the script, it is used to predict the melting point of a copper-nickel alloy.
Why does the actual melting behavior of the copper-nickel alloy differ from the linear interpolation prediction?
-The actual melting behavior of the copper-nickel alloy differs from the linear interpolation prediction because the alloy does not melt at a single fixed temperature but over a range of temperatures, indicating a more complex phase behavior than simple linear interpolation can account for.
What are the terms 'liquidus' and 'solidus' boundaries in the context of phase diagrams?
-In phase diagrams, the 'liquidus' boundary is the line above which a single liquid phase is stable, while the 'solidus' boundary is the line below which a single solid phase is stable. Between these two boundaries, both solid and liquid phases coexist.
What is the significance of the 'alpha' phase in the copper-nickel phase diagram?
-The 'alpha' phase in the copper-nickel phase diagram represents a substitution solid solution of copper and nickel. It signifies the region where both copper and nickel are mixed in the solid state, maintaining the same crystal structure.
What does the term 'substitution solid solution' imply in the context of the copper-nickel system?
-A 'substitution solid solution' implies that atoms of one element (in this case, nickel) can replace atoms of another element (copper) in the crystal lattice without changing the overall crystal structure. This is possible due to similar atomic sizes and the same crystal structure (cubic close-packed) of both elements.
How does the phase diagram of copper and nickel help in understanding the behavior of different alloys within the system?
-The phase diagram of copper and nickel helps in understanding the behavior of different alloys by showing the stable phases at various compositions (weight percent nickel) and temperatures. It provides insights into the melting points, the range of temperatures over which melting occurs, and the coexistence of solid and liquid phases.
Outlines
🔍 Introduction to Phase Diagrams
This paragraph introduces the concept of phase diagrams in the context of material science, specifically focusing on the copper-nickel alloy system. It explains the terminology used in phase diagrams, such as 'alloy' and 'system,' and provides basic facts about copper and nickel, including their crystalline structures and melting points. The paragraph sets the stage for a deeper exploration of phase diagrams by discussing the melting points of pure copper and nickel and how they relate to the phase diagram's temperature axis.
📊 Building a Phase Diagram for Copper and Nickel
The second paragraph delves into constructing a phase diagram for copper and nickel, starting with the one-dimensional phase diagrams for each element based on their melting points. It then introduces the concept of weight percent nickel as a compositional axis, allowing for the representation of various copper-nickel alloys. The paragraph discusses the assumption of linear interpolation to predict the melting point of a 50-50 alloy, highlighting the process of creating a phase diagram that includes both temperature and composition variables.
🧪 Experimental Observations in Alloy Melting
This paragraph contrasts the theoretical predictions of phase diagrams with experimental observations. It points out that while pure copper and nickel melt at specific temperatures, an alloy of copper and nickel begins melting at a lower temperature and completes at a higher one, indicating a range of temperatures over which melting occurs. The paragraph introduces the terms 'liquidus' and 'solidus' to describe the boundaries of melting for alloys, emphasizing the difference between the behavior of pure elements and alloys in phase diagrams.
📚 Understanding the Copper-Nickel Phase Diagram
The fourth paragraph provides a detailed explanation of the phase diagram for the copper-nickel system. It describes the alpha phase, a substitution solid solution of copper and nickel, and how it relates to the Hume-Rothery rules. The paragraph explains the significance of the liquidus and solidus boundaries and the region between them where both solid and liquid phases coexist. It also discusses the importance of phase diagrams in understanding the equilibrium phases at different compositions and temperatures.
📘 Defining the Equilibrium Phase Diagram
The final paragraph concludes the discussion by defining the equilibrium phase diagram. It emphasizes that a phase diagram is a representation in the space of relevant thermodynamic variables, indicating the phases in equilibrium. The paragraph connects the specific example of the copper-nickel phase diagram to the broader concept of phase diagrams, which can include other variables such as pressure or magnetic fields, and highlights the role of phase diagrams in material science.
Mindmap
Keywords
💡Phase Diagram
💡Copper-Nickel Alloy
💡Melting Point
💡Solid Solution
💡Hume-Rothery Rules
💡Liquidus Boundary
💡Solidus Boundary
💡Equilibrium
💡Interpolation
💡Latent Heat
💡Substitutional Solid Solution
Highlights
Introduction to phase diagrams as an essential topic in mechanical science.
Use of copper-nickel alloy as an example to introduce phase diagrams.
Copper and nickel both have a cubic close-packed crystalline structure.
Melting points of copper and nickel are 1085°C and 1453°C respectively.
Explanation of one-dimensional phase diagrams for copper and nickel with temperature as the variable.
Demonstration of how the melting point and solid-liquid coexistence occur at specific temperatures.
Assumption of linear interpolation for predicting the melting point of a copper-nickel alloy.
Discovery that actual melting behavior of the alloy differs from linear interpolation predictions.
Introduction of TS and TL temperatures to describe the melting range of the alloy.
Experimentation with different alloy compositions to plot TS and TL temperatures.
Definition of liquidus and solidus boundaries in phase diagrams.
Explanation of the alpha phase as a substitution solid solution of copper and nickel.
Discussion on the Hume-Rothery rules for the formation of substitution solid solutions.
Illustration of the phase diagram for the copper-nickel system with weight percent nickel on the x-axis and temperature on the y-axis.
Identification of the lance-shaped region between liquidus and solidus where both solid and liquid phases coexist.
Finalization of the copper-nickel phase diagram and its significance in material science.
Definition of an equilibrium phase diagram and its role in representing phases in equilibrium at different compositions and temperatures.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: