[H2 Chemistry] 2023 Topic 1 Atomic Structure & Physical Periodicity Lecture 1
TLDRThis chemistry lecture introduces H2 students to atomic structure and physical periodicity, guiding them through lecture organization, learning objectives, and syllabus documents. The instructor emphasizes the importance of understanding atomic particles, isotopes, and the strong nuclear force. The lecture delves into electronic configurations, subshells, and the Pauli Exclusion Principle, using the periodic table as a tool to simplify the process. It also covers exercises on isotopes, ions, and deflection of charged particles, aiming to deepen students' comprehension of atomic structure and prepare them for further studies in chemistry.
Takeaways
- 📚 The lecture notes for H2 Chemistry are organized by topic number and title, with guiding questions and learning objectives that correspond to the syllabus documents and Google Classroom topic numbers.
- 🔍 Guiding questions are designed to provoke deeper thinking about the topic before revision, and answers to these questions will become clearer as the topics are studied in depth.
- 🚀 The lecture covers fundamental particles beyond protons, neutrons, and electrons, encouraging students to explore these areas on their own for a more in-depth understanding of atomic structure.
- 📈 Learning objectives are aligned with the syllabus and are used as a checklist during revision to ensure that students have grasped the key concepts and can apply them.
- 🧲 The lecture introduces the concept of the strong nuclear force, which is responsible for holding the nucleus together despite the repulsive forces between protons.
- 🌌 A video on Quantum chromodynamics is recommended for students interested in understanding the forces at play within the atomic nucleus.
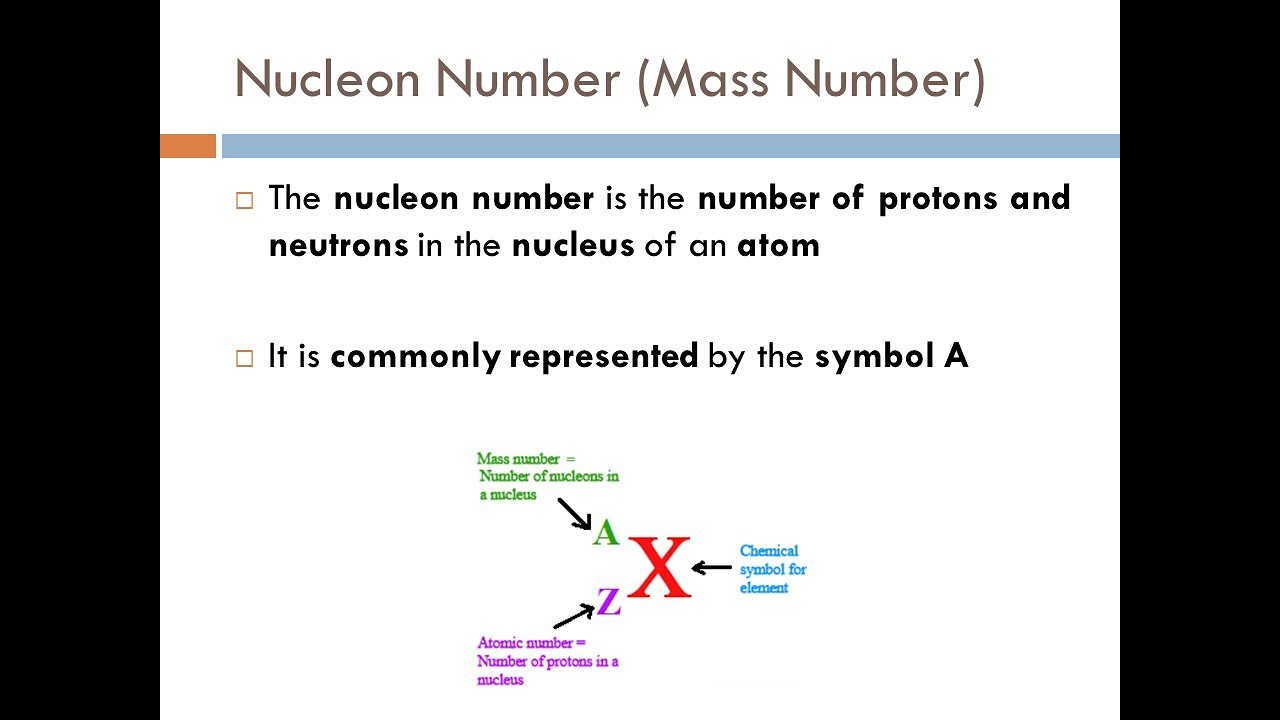
- 🔬 Isotopes are atoms of the same element with different numbers of neutrons, and they can be stable or unstable, with some having significant applications in dating techniques.
- ⚛️ The lecture explains how to calculate the relative atomic mass and distinguish between common and uncommon isotopes using the periodic table.
- 📉 The deflection of charged particles in an electric field is discussed, with the angle of deflection being directly proportional to the charge and inversely proportional to the mass of the particle.
- 🌟 The concept of electronic configuration is introduced, explaining how electrons are arranged in shells, subshells, and orbitals based on the principal quantum number.
- 📊 The importance of understanding and being able to draw the shapes of s, p, and d orbitals is highlighted, as this is a key part of the H2 Chemistry curriculum.
Q & A
What is the significance of the topic number and title in the lecture notes for H2 Chemistry?
-The topic number and title in the lecture notes serve as a guide for students to navigate through the H2 Chemistry curriculum. They correspond to the content covered in Google Sites and Google Classroom, helping students to organize their revision and study according to the structured curriculum.
Why are guiding questions included in the lecture notes?
-Guiding questions are included to prompt students to think about the topic in a deeper way before they start revising or reading through the topics. They may already have some answers based on prior knowledge, but these questions are designed to become clearer as students revise and learn more about the subject.
How do the learning objectives or outcomes in the lecture notes relate to the syllabus documents?
-The learning objectives and outcomes are written in a way that corresponds directly to the syllabus documents. This allows students to review these objectives during their revisions and ensures that they are studying the content that is expected of them in the curriculum.
Why is it important for students to understand the learning outcomes before starting their revision?
-Understanding the learning outcomes before starting revision allows students to have a clear goal in mind for what they need to achieve in their studies. It helps them to assess their current knowledge and identify areas where they need to focus their revision efforts.
What is the role of references beyond the A levels in the H2 Chemistry curriculum?
-The references beyond the A levels provide additional resources for students who wish to delve deeper into the subject matter. They offer more rigorous study materials for those interested in a comprehensive understanding of chemistry beyond the scope of the H2 curriculum.
What is the fundamental question students might ask about the nucleus of an atom?
-Students might wonder why the nucleus doesn't disintegrate on its own, considering that it contains protons with like charges that should repel each other according to the principles of electricity.
What force is responsible for holding the nucleus of an atom together?
-The strong nuclear force, also known as the strong interaction, is responsible for holding the nucleus together. This force is more powerful than electromagnetism and keeps protons and neutrons tightly bound within the nucleus.
What are Isotopes and how do they differ from each other?
-Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They can have different nuclear properties, such as stability or radioactivity, but share the same chemical properties due to having the same number of valence electrons.
How can you determine the relative atomic mass of an element?
-The relative atomic mass of an element is determined by considering the relative abundance of its isotopes and their respective masses. It is the average mass of one atom of the element compared to 1/12th the mass of one atom of carbon-12.
What is the significance of the relative atomic mass being an average value?
-The relative atomic mass is an average value because it takes into account the relative abundance of all possible isotopes of an element. This average reflects the actual distribution of isotopes found in nature.
Can you provide an example of how to calculate the relative atomic mass of an element using its isotopes and their abundances?
-Yes, for chlorine, which has isotopes氯35 and氯37 with a natural abundance of approximately 3:1 (75%氯35 and 25%氯37), the relative atomic mass is calculated as (35 * 0.75) + (37 * 0.25), which equals 35.5.
What is the difference between the electronic configuration in secondary school and in H2 Chemistry?
-In secondary school, students learn to write electronic configurations by separating the principal quantum shells with commas, such as 2,3 for carbon. In H2 Chemistry, students write electronic configurations by showing the number of electrons in each subshell, such as 1s2 2s2 2p2 for carbon.
What is the relationship between principal quantum number, subshells, and orbitals?
-The principal quantum number determines the number of subshells in an atom. Each subshell is denoted by a letter (s, p, d, f) and corresponds to a specific angular momentum quantum number. The number of orbitals in a subshell is related to the type of subshell (e.g., s subshell has 1 orbital, p subshell has 3 orbitals, etc.), and each orbital can hold a maximum of two electrons.
How do you draw the 3D representation of an s orbital?
-To draw an s orbital, start by drawing a sphere to represent the electron cloud. Then, label the axes as x, y, and z to give a three-dimensional perspective. Ensure that the 2s orbital is larger than the 1s orbital to represent the increased distance from the nucleus.
What is the significance of the Pauli Exclusion Principle in electronic configurations?
-The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that an orbital can hold a maximum of two electrons with opposite spins, which prevents electrons from occupying the same quantum state within an atom.
Why is it incorrect to insist that an electron with a principal quantum number n=2 must be in a spherical orbital?
-It is incorrect because an electron with n=2 could occupy either a 2s or 2p orbital. The 2s orbital is spherical, but the 2p orbitals are dumbbell-shaped. Since the electron could be in either type of orbital, we cannot insist that its orbital must be spherical.
How can the periodic table be used to easily determine the electronic configuration of elements?
-The periodic table can be used to determine electronic configurations by understanding the blocks (s, p, d, f) and moving along the 'corridors' of elements within a period. Each block corresponds to a type of subshell, and the position in the period indicates the principal quantum number and the number of electrons in each subshell.
What are the exceptions to the typical electronic configurations in the periodic table?
-Chromium and copper are exceptions to the typical electronic configurations. Chromium has an electronic configuration of [Ar] 3d5 4s1, while copper has [Ar] 3d10 4s1. These configurations are due to the stability provided by half-filled or fully-filled subshells and the concept of exchange energy.
How do you write the electronic configuration for ions?
-For cations, write the electronic configuration of the neutral atom first and then remove the number of electrons equal to the positive charge, starting from the highest energy level. For anions, add the extra electrons to the valence orbital.
What is the definition of a transition metal, and why are scandium and zinc not considered transition metals?
-A transition metal is a d-block element that forms one or more stable ions with partially filled d subshells. Scandium and zinc are not considered transition metals because scandium typically forms a stable ion with a d0 configuration (Sc3+), and zinc forms a stable ion with a d10 configuration (Zn2+).
What is the electronic configuration for the ground state of an element in group 13 with three electrons of the highest energy?
-The electronic configuration for the ground state of an element in group 13 with three electrons of the highest energy corresponds to gallium (Ga), which is 4s2 3d10 4p1.
Outlines
📚 Introduction to H2 Chemistry Lecture
The lecturer begins by introducing the structure of the H2 chemistry lecture notes, highlighting the organization by topic number and title. The notes are designed to assist students in their revision, with guiding questions and learning objectives aligned with the syllabus documents. The lecturer emphasizes the importance of understanding the content deeply and provides additional resources for students who wish to explore beyond the curriculum. The lecture then transitions into a discussion on atomic structure and physical periodicity, with a focus on the fundamental particles that make up atoms.
🔬 Atomic Structure and Subatomic Particles
The lecture delves into the atomic structure, discussing the discovery of subatomic particles such as electrons, protons, and neutrons. It provides a historical background, mentioning experiments by scientists like John Dalton, JJ Thompson, and Rutherford that contributed to our understanding of the atom. The lecturer explains the concept of isotopes and introduces the strong nuclear force that holds the nucleus together, despite the repulsive forces between protons. The lecture also includes a video on quantum chromodynamics to further explain the forces at play within the atomic nucleus.
🧬 Isotopes and Their Properties
The lecture continues with a discussion on isotopes, which are atoms of the same element with different numbers of neutrons. It explains how isotopes can be stable or unstable, with examples like carbon-14 used in archaeological dating. The lecturer provides examples of common isotopes for hydrogen, carbon, oxygen, and sulfur, and discusses how the nuclear number influences their properties. The lecture also includes exercises for students to practice calculating the number of protons, neutrons, and electrons in various particles.
🚀 Deflection of Charged Particles and Isotope Identification
This section introduces the concept of ion deflection in an electric field, explaining how the charge and mass of particles affect their deflection angles. The lecturer provides a formula to calculate the angle of deflection and emphasizes the importance of understanding the proportionality constant in such calculations. The lecture includes an exercise where students determine the deflection angles for various particles, considering their charge and mass. Additionally, the lecturer discusses how to identify common isotopes using the periodic table and the concept of relative atomic mass.
🔡 Electronic Configuration and Quantum Numbers
The lecturer introduces the concept of electronic configuration, explaining the arrangement of electrons in atoms. It discusses the principal quantum number, subshells, and the distribution of electrons in s, p, and d orbitals. The lecture emphasizes the importance of understanding the shape and orientation of these orbitals, as well as the maximum number of electrons each subshell can hold. The lecturer also explains the significance of the Pauli Exclusion Principle and Hund's Rule in determining the configuration of electrons in atoms.
🌐 Periodic Table and Electronic Configurations
The lecture focuses on using the periodic table to determine the electronic configuration of elements. It explains the blocks of the periodic table (s, p, d, and f blocks) and how they relate to the electron configuration. The lecturer demonstrates how to systematically fill the orbitals by moving across the periods and groups of the periodic table. This method simplifies the process of writing electronic configurations without memorizing the Aufbau principle or energy levels.
🚫 Exceptions to Electronic Configuration Rules
This section discusses exceptions to the standard electronic configurations, such as chromium and copper, which do not follow the expected filling order due to the stability provided by half-filled or fully-filled subshells. The lecturer explains the concept of exchange energy and how it affects the electronic configuration, leading to exceptions like Cr having a 3d5 4s1 configuration and Cu having a 3d10 4s1 configuration instead of the expected 3d4 4s2 for both.
🗓️ Transition Metals and Their Electronic Configurations
The lecture concludes with a brief introduction to transition metals, defined as d-block elements that form stable ions with partially filled d-subshells. It clarifies that scandium and zinc are not considered transition metals because they form ions with either a d0 or d10 configuration. The lecturer also addresses the importance of understanding the ground state electronic configurations for elements in the periodic table and provides an exercise to identify the configuration of the highest energy electrons for group 13 elements.
Mindmap
Keywords
💡Atomic Structure
💡Physical Periodicity
💡Subatomic Particles
💡Isotopes
💡Electronic Configuration
💡Ions
💡Periodic Table
💡Relative Atomic Mass
💡Transition Elements
💡Quantum Chromodynamics
💡Hund's Rule
💡Pauli Exclusion Principle
Highlights
Introduction to H2 Chemistry lecture on atomic structure and physical periodicity.
Explanation of lecture note organization and correlation with Google Sites and Google Classroom.
Use of guiding questions to deepen understanding of atomic structure topics.
Importance of learning objectives in aligning with the syllabus documents for effective revision.
Inclusion of learning objectives from other topics in the syllabus for a comprehensive understanding.
Suggestion to explore fundamental particles and quantum chromodynamics for deeper knowledge.
Discussion on the strong nuclear force and its role in holding the nucleus together.
Introduction to isotopes and their distinction based on neutron numbers.
Explanation of nuclear properties related to stability and instability of isotopes.
Exercise on calculating the number of protons, neutrons, and electrons in various particles.
Understanding the deflection of charged particles in an electric field and its relation to mass and charge.
Quantifying the masses of atoms through the concept of relative atomic mass.
Calculation of relative atomic mass using the relative abundance of isotopes.
Introduction to electronic configuration and its significance in chemistry.
Explanation of principal quantum numbers, shells, and subshells in electronic configuration.
Drawing and understanding the shapes of s, p, and d atomic orbitals.
Application of the periodic table in determining electronic configurations efficiently.
Discussion on exceptions to the typical electronic configurations, such as chromium and copper.
Writing electronic configurations for ions by adjusting for charge.
Transition metals defined and distinguished within the periodic table.
Upcoming lecture topics on periodic trends in atomic and ionic radii.
Transcripts
Browse More Related Video

Learning Outcomes (f), (g), (h) of Atomic Structure [JC H2 Chemistry]

Cram AP Chem Unit 1: Atomic Structures and Properties
Learning Outcomes (d) and (e) from Atomic Structure - JC H2 Chemistry

15.1 Introduction to Nuclear Chemistry | High School Chemistry

25. Quantum Mechanics VII: Summary of postulates and special topics

Questions to Learning Outcomes (f), (g) & (h) of Atomic Structure from JC H2 Chemistry Syllabus
5.0 / 5 (0 votes)
Thanks for rating: