Molarity Practice Problems (Part 2)
TLDRThis educational video script teaches the concept of molarity, a key tool in chemistry for converting between the volume of a solution and the amount of solute in moles. It walks through several problems, demonstrating how to use molarity as a conversion factor. The script covers unpacking molarity to create usable conversion factors, converting between liters and moles, and additional steps involving unit conversions such as grams to moles. It emphasizes the importance of understanding molarity as moles per liter and the flexibility to flip conversion factors when necessary, providing a clear guide for solving molarity-related problems.
Takeaways
- 🧪 Molarity is a concentration measurement that indicates the number of moles of solute per liter of solution.
- 🔢 To convert liters of a solution to moles, you can use molarity as a conversion factor by 'unpacking' it to moles over liters.
- 🔄 When molarity is given, it is essential to expand it to understand that it means 1.5 moles per every one liter of solution, for example.
- 📚 The script demonstrates how to use molarity to convert between liters and moles in a solution, which is crucial for solving chemistry problems.
- 📉 To find the volume of a solution given a certain number of moles of solute, you can flip the molarity conversion factor to get liters over moles.
- ⚖️ When starting with grams of a substance, you must first convert grams to moles using the molar mass before applying molarity to find the volume.
- 📏 For volumes less than a liter, it's often more practical to convert liters to milliliters for a more precise and understandable measurement.
- 🔄 If the units required for the problem are not directly provided by the molarity conversion factor, it may be necessary to flip the factor to make it work.
- 📐 The script shows that molarity can be used in conjunction with other unit conversions, such as from milliliters to liters, to solve complex problems.
- 📝 The process of solving molarity problems involves unpacking and flipping conversion factors, and using molar mass to convert between grams and moles.
- 📚 The importance of understanding and applying molarity as a conversion factor is emphasized, as it is a fundamental concept in chemistry for solving problems involving solutions.
Q & A
What is molarity and how is it used as a conversion factor in the context of the video?
-Molarity is a measure of the concentration of a solute in a solution, expressed in moles of solute per liter of solution. In the video, molarity is used as a conversion factor to go between liters of a solution and moles of solute within that solution.
How many moles of NaCl are in 3.5 liters of a 1.5 molar solution according to the video?
-To find the moles of NaCl in 3.5 liters of a 1.5 molar solution, you multiply the volume of the solution (3.5 liters) by the molarity (1.5 moles/liter), resulting in 5.25 moles of NaCl.
What does the term 'unpacking' refer to in the context of using molarity as a conversion factor?
-'Unpacking' refers to the process of expressing molarity in a way that can be used as a conversion factor. For example, 1.5 molar can be 'unpacked' to mean 1.5 moles per liter, which can then be used to convert liters to moles or vice versa.
How can you determine the final volume of a solution if you have 4.1 moles of glucose and want to make a 0.25 molar solution?
-To determine the final volume of a 0.25 molar glucose solution starting with 4.1 moles, you 'unpack' the molarity to get 0.25 moles per liter, then flip it to liters per 0.25 moles. Dividing the moles of glucose by this factor gives the volume in liters.
What is the molar mass of FeCl3 and how is it used in the video to convert grams to moles?
-The molar mass of FeCl3 is calculated by adding the molar mass of iron (Fe) and three times the molar mass of chlorine (Cl). In the video, this molar mass is used as a conversion factor to convert 35.0 grams of FeCl3 to moles by dividing the grams by the molar mass.
How do you convert milliliters to liters for the purpose of using molarity as a conversion factor?
-To convert milliliters to liters, you divide the number of milliliters by 1000, since there are 1000 milliliters in one liter. This conversion is used in the video to change 725 milliliters to liters before using molarity to find moles.
What is the molar mass of NaOH and how is it used in the video to convert moles to grams?
-The molar mass of NaOH is the sum of the atomic masses of sodium (Na), oxygen (O), and hydrogen (H), which equals approximately 40 grams per mole. In the video, this molar mass is used to convert 1.8 moles of NaOH to grams by multiplying the moles by the molar mass.
Why might it be more appropriate to express a small volume in milliliters rather than liters?
-Expressing a small volume in milliliters is more appropriate because it provides a more precise and understandable measure for volumes less than one liter, as shown in the video when converting 0.14 liters to 140 milliliters.
What is the significance of 'flipping' the conversion factor when using molarity?
-Flipping the conversion factor is significant when the units you need to cancel out are not in the correct position. By flipping the factor, you can ensure that the units you want to remain (like liters or moles) are on the correct side of the equation.
Can you provide an example of a step-by-step process for solving molarity problems as demonstrated in the video?
-An example process would be: 1) Convert the given volume from milliliters to liters if necessary. 2) Use molarity to convert the volume to moles. 3) If required, convert moles to grams using the molar mass of the solute. 4) Flip or unpack the molarity as needed to create the correct conversion factors.
Outlines
🧪 Conversion of Molarity to Moles and Liters
This paragraph introduces the concept of using molarity as a conversion factor to determine the number of moles in a solution given its volume and molar concentration. It explains the process of converting 3.5 liters of a 1.5 molar NaCl solution into moles by using the molarity as a conversion factor. The key takeaway is that molarity, expressed as moles per liter, can be 'unpacked' and used to find the moles of solute in a given volume of solution. The example demonstrates that 3.5 liters of a 1.5 molar NaCl solution contains 5.3 moles of NaCl when rounded to two significant figures.
📚 Calculating Solution Volume from Moles and Molarity
The second paragraph discusses how to calculate the final volume of a solution when given the number of moles of a solute and the desired molarity. It uses the example of creating a 0.25 molar glucose solution from 4.1 moles of glucose. The explanation clarifies that molarity must be 'unpacked' into a conversion factor to switch between moles and liters. The process involves flipping the molarity ratio to have liters on the top and moles on the bottom, resulting in a final solution volume of 16 liters when the calculation is completed.
🧪 Preparing a Molar Solution from Grams of Solute
This paragraph delves into a more complex problem where the goal is to find the volume of a 1.5 molar FeCl3 solution prepared from 35.0 grams of iron chloride. The explanation outlines the necessary steps: converting grams to moles using the molar mass of FeCl3, then using molarity to find the volume in liters, and finally converting liters to milliliters for a more appropriate volume unit. The process involves 'unpacking' and flipping conversion factors as needed, resulting in a final solution volume of 140 milliliters.
📈 Determining Grams of Solute for a Given Molar Solution
The final paragraph explains how to calculate the mass of NaOH needed to prepare a 2.5 molar solution in 725 milliliters. It details a three-step process: converting milliliters to liters, using molarity to find the moles of NaOH, and then converting moles to grams using the molar mass of NaOH. The summary emphasizes the importance of 'unpacking' molarity into a usable conversion factor and demonstrates the calculations leading to the requirement of 72 grams of NaOH to achieve the desired solution concentration.
Mindmap
Keywords
💡molarity
💡conversion factor
💡moles
💡liters
💡solute
💡solvent
💡molar mass
💡significant figures
💡milliliters
💡chemical compounds
Highlights
Molarity can be used as a conversion factor to go between liters and moles in a solution.
To use molarity as a conversion factor, it must be expanded or 'unpacked' to show moles over liters.
Example problem: Calculating moles of NaCl in 3.5 liters of a 1.5 molar solution.
Molarity of 1.5 molar means there are 1.5 moles per liter of solution.
Multiplying the volume in liters by the molarity gives the number of moles.
Conversion factor may need to be flipped to match units for the calculation.
Example: Calculating the final volume of a 0.25 molar glucose solution with 4.1 moles of glucose.
Molarity can be used to convert moles to liters and vice versa.
Problem involving converting grams of FeCl3 to moles and then using molarity to find the solution volume.
Molar mass is used to convert grams to moles when the amount of substance is given in grams.
Conversion from grams to moles requires flipping the molar mass to moles over grams.
Using molarity to convert moles to liters involves setting up a conversion factor with liters over moles.
Problem: Determining the volume of a 1.5 molar FeCl3 solution made from 35.0 grams of FeCl3.
Conversion from liters to milliliters may be necessary for reporting small volumes.
Example: Making a 2.5 molar NaOH solution with a specific number of grams.
Steps include converting milliliters to liters, using molarity to find moles, and then converting moles to grams.
Molar mass of NaOH is used to convert moles to grams for the final calculation.
Always remember to expand and unpack molarity to use it as a conversion factor.
Transcripts
Browse More Related Video

Molarity Practice Problems

Finding Grams and Liters Using Molarity - Final Exam Review

How to Do Solution Stoichiometry Using Molarity as a Conversion Factor | How to Pass Chemistry

Molarity - Chemistry Tutorial
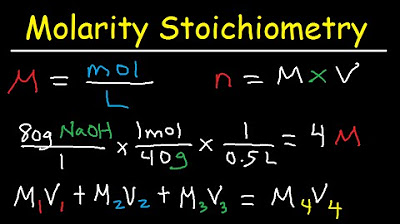
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry

Concentration and Molarity: The Key to Chemical Solutions
5.0 / 5 (0 votes)
Thanks for rating: